Background and aims
The aetiopathogenesis of rheumatoid arthritis (RA) is only partially understood, but is believed to result from a multi-step process, whereby in genetically susceptible individuals, environmental factors induce a pathological activation of the immune system that may eventually lead to systemic autoimmunity and subsequent clinical onset of the disease.1 Current evidence suggests that the immune onset of RA takes place outside of the joints several years before clinical manifestations and that primed memory T cells migrate from the peripheral blood into the synovial joints, where they are probably activated by cross-reactivity with auto-antigens expressed in joints and clonally expand. Indeed, expanded T cell clones can be found in the synovial tissue of established RA patients.2,3 The aim of this study was to examine if expanded T cell clone signatures can be detected in the peripheral blood before the development of clinical RA.
Methods
Next-generation sequencing of the T Cell Receptor β (TCRβ) complementarity-determining region 3 (CDR3) repertoire was performed on genomic DNA isolated from blood samples of individuals genetically at risk for RA, namely first-degree relatives of RA patients (RA-FDR) at different preclinical phases of disease development (SCREEN-RA cohort),4 and of matched RA patients used as a control group (SCQM cohort).5,6 The European League Against Rheumatism (EULAR) recommendations for terminology were used to categorise RA-FDR in preclinical RA stages.7 All individuals were matched for age and sex, and categorised into four groups (n=20/group): Group 1: ‘healthy’ asymptomatic RA-FDR without autoantibodies and symptoms associated with possible RA. Group 2: Asymptomatic RA-FDR with evidence of ‘systemic autoimmunity associated with RA’ defined by high levels of anti-citrullinated peptide antibodies (≥3 times the upper limit of normal of the ELISA test). Group 3: RA-FDR having presented undifferentiated arthritis (n=8) or having developed classifiable RA after inclusion (RA-converters, n=12). Group 4: patients with established RA of <3 years duration. T cell clones were characterised by their unique TCRβ CDR3 sequence and their degree of expansion (frequency). Clones with a frequency >0.5% were considered to be highly expanded clones (HEC). Both absolute number and frequency of productive T cell clones was compared between the four groups using mixed effect regression models to account for matching.
Results
Expanded circulating TCR clones (>0.1%) tended to occur more frequently in patients in later preclinical stages of RA or with established RA (Figure 1A). Specifically, the absolute number of HEC was significantly higher in RA patients (mean 4.65%) and tended to be higher in symptomatic RA-FDR (mean 3.4%) compared to ‘healthy’ RA-FDR (mean 1.55%, p=0.003 and p=0.07, respectively) (Figure 1B). Asymptomatic at-risk individuals with strong RA-associated systemic autoimmunity did not differ from ‘healthy’ RA-FDR in terms of absolute number and frequency of clones. Finally, the number of HEC tended to be slightly higher around the time of RA onset, but specific clones were not shared within or between the different groups (data not shown).
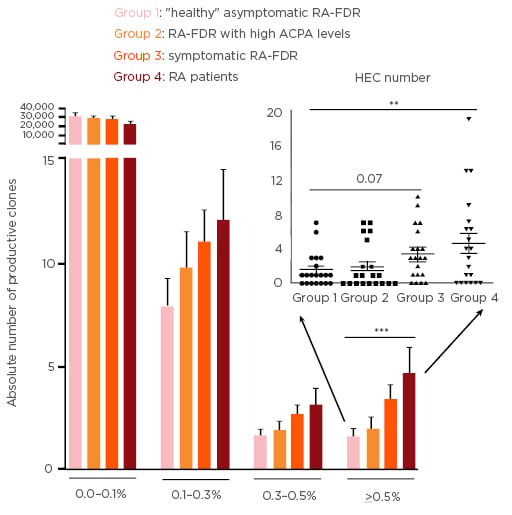
Figure 1: Absolute number of productive T cell receptor clones by clonal size.
A) Bars show mean and SEM for clones at different frequency cut-offs. (B) Each dot represents the number of HEC observed for one individual (group mean and SEM are shown as line and error bars). CPA: anti-citrullinated peptide antibodies; HEC: highly expanded clones; SEM: standard error of the mean; RA: rheumatoid arthritis; RA-FDR: first-degree relatives of RA patients.
**p<0.01, ***p<0.001 using a mixed effect regression model to account for matching.
Conclusions
Highly expanded T cell clones were detected in the peripheral blood of at-risk individuals before the clinical onset of RA, particularly in the later preclinical phases of RA development. Tracking these dominant T cell clones in longitudinal analyses and elucidating their role might help to better understand the earliest pathogenic events in RA.








