BACKGROUND
Glucocorticoids (GC) affect the Wnt signaling pathway, one of the factors involved in the development of glucocorticoid-induced osteoporosis (GIOP). The authors previously reported that GC therapy increased serum sclerostin, an inhibitor of Wnt signaling, and decreased Wnt3a, a ligand for Wnt signaling, suggesting the potential of inhibiting sclerostin as a treatment for GIOP.1,2 The authors recently demonstrated the efficacy of romosozumab (ROMO), an antibody against sclerostin, in patients newly started on GCs over a 12-month period.3 However, the long-term efficacy of the sequential use of ROMO and denosumab (DMAb) is uncertain. The aim of this study was to compare the efficacy of 12 months of ROMO and 24 months of sequential therapy with DMAb (ROMO-DMAb), with that of DMAb or bisphosphonates (BP) alone in patients newly started on GCs.4
METHODS
This was a randomised, open-label, prospective study. Patients with rheumatic disease who had not previously received osteoporosis drugs and were newly treated with prednisolone ≥15 mg/day were randomly assigned to receive ROMO-DMAb, DMAb, or risedronate. Over a 36-month period, the authors measured bone mineral density (BMD) in the lumbar spine, femoral neck, and total hip every 6 months. After the 12-month restricted period of ROMO, patients in the ROMO-DMAb group were switched to DMAb. The authors also measured bone turnover markers and Wnt signaling-related molecules every 3 months, and assessed the incidence of new fractures and adverse events.
RESULTS
Eleven patients were assigned to the ROMO-DMAb group, 14 to the DMAb group, and 14 to the BP group. The median age of all three groups was in the 70s (between 53–92 years), mostly females, who were all postmenopausal. The median daily prednisolone dose ranged from 15.0 to 25.0 mg, with no significant differences among the three groups. The cumulative GC doses at 12- and 36-month were also not significantly different among the three groups. The median (25th–75th percentile) percentage change in lumbar spine BMD from baseline at 36-month was greatest in the ROMO-DMAb group (ROMO-DMAb: 11.3% [6.8–13.8]; DMAb: 9.4% [4.7–13.5]; BP: 2.1% [-1.3–6.8]; Figure 1). At 36-month, the median percentage change in total hip BMD was also greatest for the ROMO-DMAb group (ROMO-DMAb: 0.99% [-4.0–5.4]; DMAb: 0.27% [-3.7–4.2]; BP: -0.61% [-7.6–5.0]), and the median percentage change in femoral neck BMD was greatest for the DMAb group (ROMO-DMAb: 1.4% [-3.7–4.2]; DMAb: 1.6% [-5.1–7.8]; BP: -2.5% [-11.0–1.9]). Serum levels of N-terminal propeptide of Type I procollagen, a bone formation marker, were lower than baseline from Month 3 in all three groups. In the ROMO-DMAb group, the rate of decrease was smallest up to Month 12, followed by a rapid decrease from Month 15 onwards, coinciding with the switch to DMAb. Serum tartrate-resistant acid phosphatase isoform 5b, a bone resorption marker, decreased in all groups. Urine pentosidine, a bone quality marker, decreased in all groups. Serum Dickkopf-1 (Dkk-1), an inhibitor of Wnt signaling, and Wnt3a in the ROMO-DMAb group decreased until Month 12, then increased from Month 15 onwards. The incidence of new fractures was not statistically different among the three groups. No patients developed cardiovascular events.
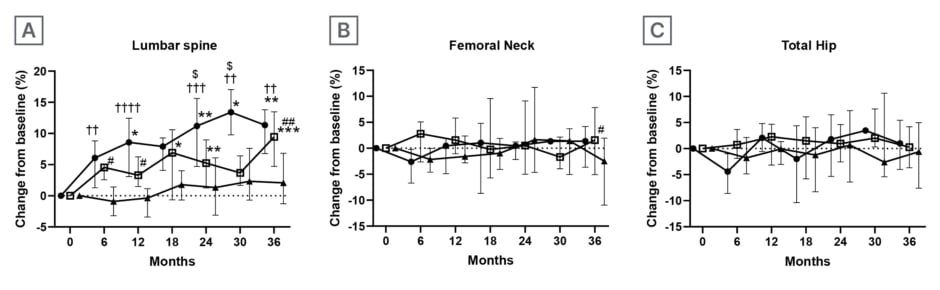
Figure 1: Percentage changes in bone mineral density.
Data are expressed as medians with interquartile ranges.
*p<0.05, **p<0.01, ***p<0.001; change within each treatment group from baseline by Dunn’s multiple comparison test.
$ROMO versus DMAb ($p<0.05), †ROMO versus BP (††p<0.01, †††p<0.001, ††††p<0.0001), #DMAb versus BP
(#p<0.05, ##p<0.01) by Tukey’s multiple comparison test.
BP: bisphosphonates; DMAb: denosumab; ROMO: romosozumab.
CONCLUSION
ROMO was efficacious in patients newly initiating GCs. Furthermore, sequential treatment with ROMO followed by DMAb demonstrated the efficacy of this GIOP treatment strategy.







