BACKGROUND
Digital ulcers (DU) affect approximately 50% of patients with systemic sclerosis (SSc), causing significant pain and disability.1,2 Current management involves both systemic and local therapies. However, the burden in terms of pain and quality of life due to refractory DUs remains heavy. Indeed, over 67% of patients with SSc experience >5 DUs in their disease course, reflecting the high burden of this disease domain.3 While selexipag, an oral selective IP prostacyclin receptor agonist,4 is approved for the treatment of SSc-associated pulmonary arterial hypertension, its potential in healing DUs is largely unexplored.5-9 The authors aimed to evaluate the long-term efficacy of selexipag, compared to iloprost, in treating SSc-DUs.
METHODS
This retrospective, multicentre study included 96 patients with SSc and refractory DUs: 32 treated with selexipag (median dose of 1,600 mg/day; interquartile range: 1,100 mg) and 64 with intravenous iloprost (0.5–2 ng/kg/min), matched for gender, disease subset, and age at diagnosis. Both groups were concomitantly treated with conventional vascular therapies (i.e., calcium channel blockers; endothelin receptor antagonists; and phosphodiesterase 5- inhibitors). The number of DUs, ischaemic pain, and Raynaud’s phenomenon (RP) severity were assessed at baseline, 6,12, and 24 months. Pain and RP were evaluated using the Likert Pain Scale (LPS) and Raynaud Condition Score (RCS), respectively. Additionally, DUs recurrence and new onset were recorded. Healing rates were estimated using Kaplan-Meier analysis.
RESULTS
DUs healing rate was significantly higher in patients treated with selexipag compared to those treated with iloprost (87% versus 28%) at 96 weeks, with the former achieving faster healing (75% versus 18% by Week 40; p<0.001; Figure 1A). The number of DUs, as well as RCS and LPS scores, showed significant improvement in the selexipag-treated group compared to the iloprost group (p<0.001 for all) throughout the 24-month follow-up (Figure 1B–D). Repeated measures analysis demonstrated significant changes over time for all three outcomes. The difference in treatment effect was supported by Mann-Whitney U analyses, showing comparable baseline measures (DUs: p=0.901; RCS: p=0.561; LPS: p=0.708); however, significant differences emerged by 6 months (DUs: p=0.001; RCS: p<0.001; LPS: p<0.001) and were maintained through 12 and 24 months (all p<0.001). Additionally, patients treated with selexipag experienced significantly lower relapse rates (5% versus 45% at 24 months; p<0.001; Figure 1E). Consistently, DUs formation remained lower with selexipag (5% versus 40% at 24 months; p<0.001; Figure 1F). Only two patients had to discontinue selexipag due to mild intolerance after 12 months of treatment.
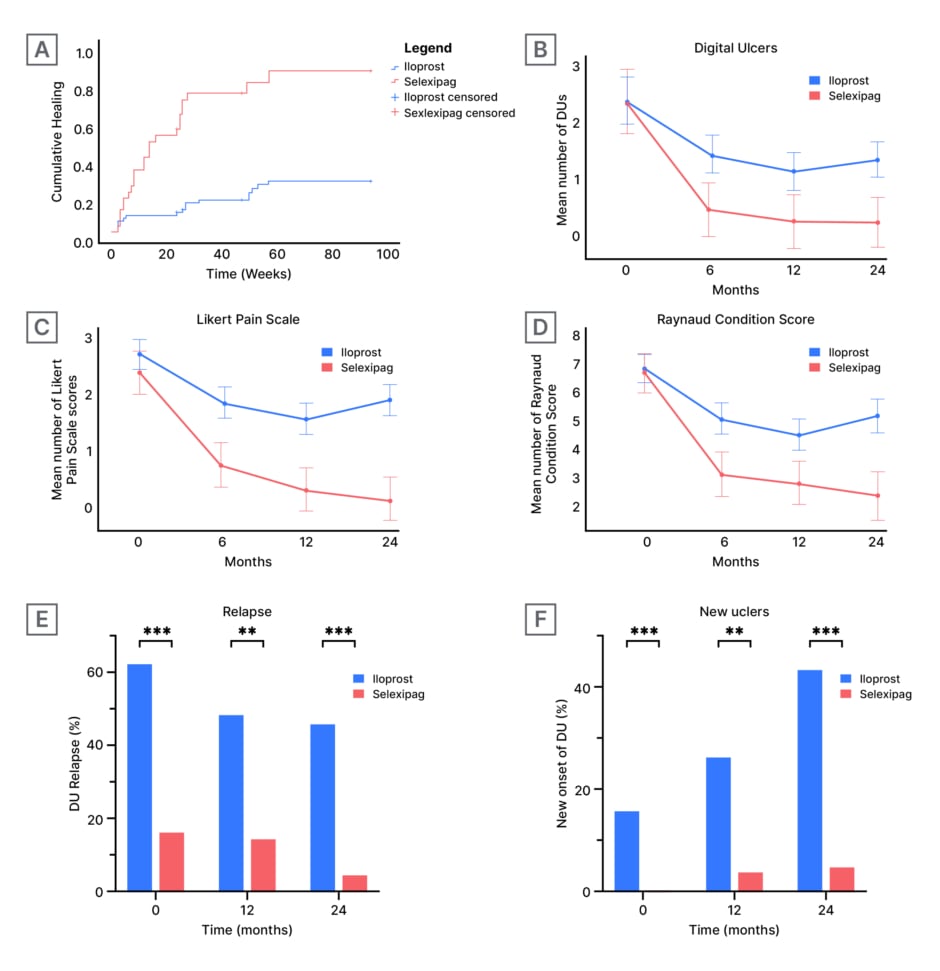
Figure 1: Comparison of treatment outcomes between iloprost and selexipag treatment groups across multiple clinical parameters over a 24-month study period.
A) Shows cumulative healing rates over time, measured in weeks.
B), C), and D) Show repeated measures analysis at 0, 6, 12, and 24-month intervals, with error bars representing standard error or CIs. B) Tracks the number of digital ulcers. C) Measures pain scores using a Likert scale. D) Evaluates Raynaud condition scores.
E) and F) Present bar chart comparisons at 6, 12, and 24 months; where E) shows relapse percentages, and F) displays the number of new ulcers per patient.
Double asterisks (**) indicate statistically significant differences between groups (p<0.01). Triple asterisks (***) indicate statistically significant differences between groups (p<0.001).
DU: digital ulcers.
CONCLUSION
This study shows that patients with SSc who are treated with selexipag have a better sustained outcomes for DUs, compared to those treated with intravenous iloprost over 24 months. Furthermore, the oral administration and good tolerability profile of selexipag make it a promising alternative option to standard therapy for SSc-related DUs, overcoming challenges such as difficult venous access, high hospitalisation costs, and lost work productivity associated with IV administration. Prospective studies are needed to confirm a wider use of selexipag other than pulmonary arterial hypertension.







