BACKGROUND AND AIMS
Early intervention in psoriatic arthritis (PsA) is disease-modifying, and delays in diagnosis, even by 6 months, can reduce treatment response. Yet, PsA remains underdiagnosed; 15% of patients with psoriasis have undiagnosed PsA, and many are diagnosed years after symptom onset. Psoriasis is typically diagnosed by general practitioners or dermatologists, while PsA diagnosis and management fall under rheumatologists. A key challenge for general practitioners and dermatologists is identifying patients who need referral to rheumatology. Rheumatologists then confirm the diagnosis and assess prognosis.
The authors1 aimed to: 1) develop and internally validate a model to identify patients suitable for rheumatologist referral; 2) develop and validate a prognostic model for PsA in patients with subclinical PsA; and 3) identify predictors of PsA.
METHODS
The authors analysed data from the Stockholm Psoriasis Cohort, a cohort study of 672 adults with new-onset psoriasis. Participants were followed clinically at 5 and 10 years, and data were also linked to administrative health registers. Predictors were collected at baseline, and outcomes were PsA diagnoses during follow-up.
The authors used recursive partitioning to develop a model for identifying patients with concomitant PsA. For prognostic modelling in subclinical PsA, they used lasso penalised logistic regression. The dataset was split into training (70%) and validation (30%) sets. To identify PsA predictors, logistic regression adjusted for age and sex was used.
RESULTS
All 672 participants were included. Over 9,255 person-years, 195 participants (29.0%) developed PsA, an incidence of 21.1 per 1,000 person-years.
The referral model, based on recursive partitioning, identified five terminal nodes using four variables: current arthralgia, human leukocyte antigen B27 (HLA-B27), number of painful body sites in the past year, and high-sensitivity C-reactive protein (hs-CRP). The model had an area under curve of 0.804, and showed good calibration (Figure 1A, C, and E).
The prognostic model included 138 participants with subclinical PsA. Eight predictors were identified: HLA-B27, uveitis history, axial and peripheral entheseal pain, male sex, tender joint count, hs-CRP, and visual analogue scale (VAS)-health. The area under the curve was 0.804, with satisfactory calibration, though it slightly underestimated risk in the highest quintile (Figure 1B, D, and F).
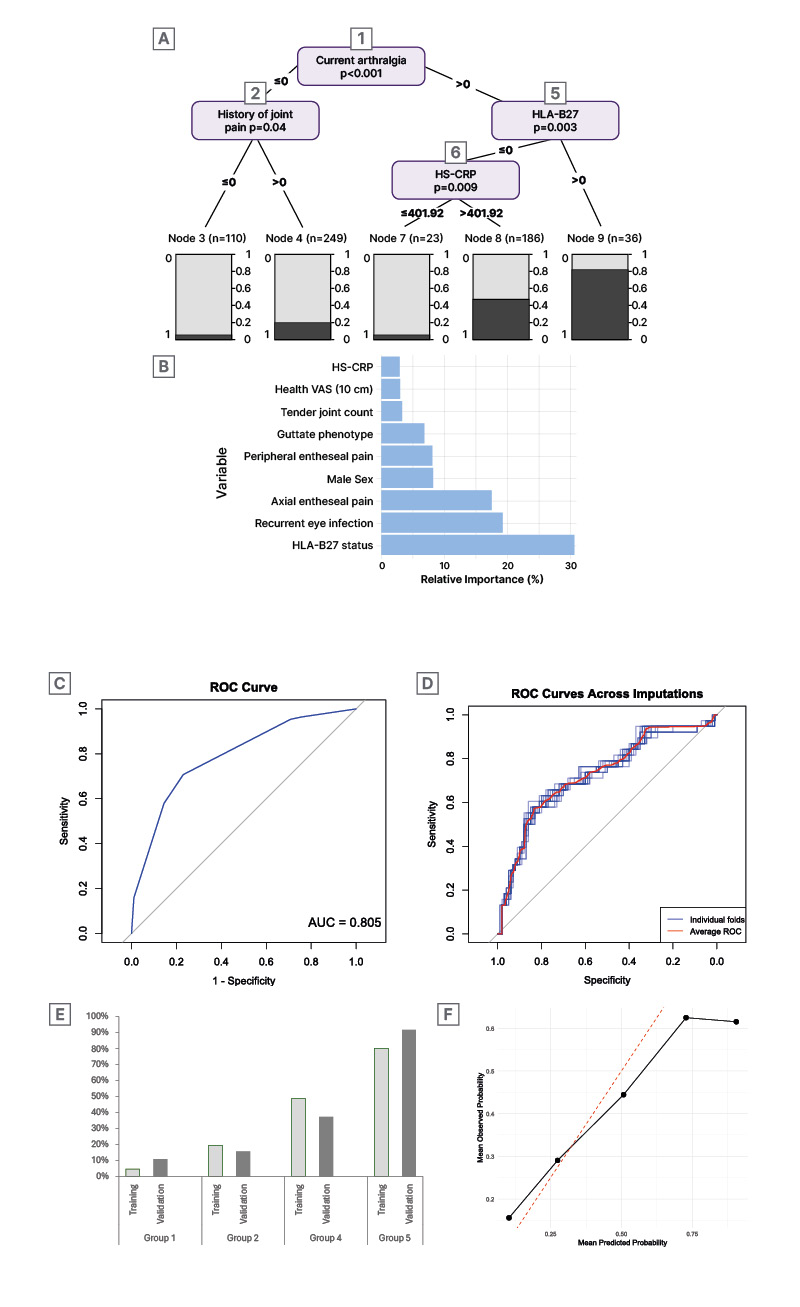
Figure 1: Prediction models for the development of psoriatic arthritis in patients with new-onset psoriasis.
A) Presents the tree-based model for the development of PsA among all patients. B) Presents the relative importance of the predictors from the logistic regression model for the development of PsA in patients with subclinical PsA. C) Presents the ROC curve for the tree-based model for all patients. D) Presents the ROC curve for the model in patients with subclinical PsA. E) Presents predicted versus observed PsA probability for the identified groups from the model in all patients. F) Presents the mean predicted and observed probabilities by predicted quintile of risk from the model in patients with subclinical PsA.
AUC: area under the curve; HLA-B27: human leukocyte antigen B27; HS-CRP: high-sensitivity C-reactive protein;
PsA: psoriatic arthritis; ROC: receiving operating characteristics; VAS: visual analogue scale.
To identify predictors, we analysed 647 participants without prior PsA. Of 48 predictors, 24 were significantly associated with PsA. Clinical variables (arthralgia, fatigue, BMI), systemic inflammation markers (serum amyloid A (SAA), hs-CRP, glycosylated acetyls), lipid markers, and genotypes (HLA-B27, human leukocyte antigen-Cw6 [HLA-Cw6]) were relevant (Table 1).
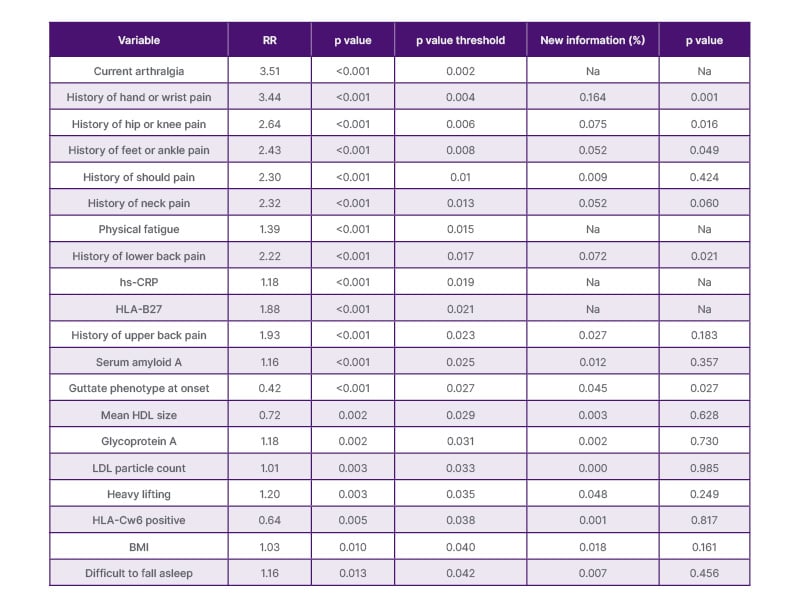
Table 1: Nineteen risk markers for psoriatic arthritis in patients with new-onset psoriasis.
This table represents 19 markers with the highest relative risk of developing psoriatic arthritis in 647 patients who were free of psoriatic arthritis at psoriasis onset, adjusted for sex and age. Lipoproteins were measured using nuclear magnetic resonance spectroscopy from LabCorp (Burlington, North Carolina, USA). hs-CRP was measured using assays from Meso scale Discovery (Rockville, Maryland, USA).
HDL: high density lipoprotein; HLA: human leukocyte antigen; HLA-B27: human leukocyte antigen B27; HLA-Cw6: human leukocyte antigen-Cw6; LDL: low density lipoprotein; hs-CRP: high-sensitive C-reactive protein; Na: not applicable; RR: relative risk.
CONCLUSION
The authors developed and internally validated two models to aid the early diagnosis and prognosis of PsA. Both models showed good discrimination and calibration.







