Interview Summary
Rheumatoid arthritis (RA) is a chronic, immune-mediated inflammatory disease that remains challenging to diagnose early and control sustainably. In this interview, rheumatologist Rieke Alten, Department of Internal Medicine and Rheumatology, Schlosspark-Klinik, University Medicine Berlin, Germany, reflects on the shift from reactive symptom suppression to proactive, treat-to-target care aimed at achieving sustained remission or low disease activity. She discusses how the timely use of targeted therapies, including TNF inhibition with etanercept, has transformed daily functioning for many patients, whilst underscoring the ongoing need to minimise long-term glucocorticoid exposure. Alten highlights practical points concerning patient selection, safety screening, and adherence, as well as the value of systematically tracking pain, function, and fatigue. She also comments on the broader access through biosimilar competition, stresses the importance of training younger colleagues who have not experienced pre-biologic disease severity and damage, and points to the extent of thoughtful tapering in sustained remission. A vivid before-and-after image from her clinic captures the era: a 30-bed ward in the 1990s with 29 patients in wheelchairs, compared with a modern clinic where only one out of 30 patients might use a wheelchair, often for non-RA reasons.INTRODUCTION
RA is a chronic, heterogeneous, autoimmune condition characterised by persistent synovitis, pain, fatigue, and progressive functional impairment.1 Delays in diagnosis and treatment can accelerate structural damage and disability, with substantial consequences on quality of life and economic productivity for patients, families, and health systems.1 Beyond joint symptoms, extra-articular manifestations and comorbidities add complexity to long-term care.1
Over the past two decades, the combination of treat-to-target strategies and targeted therapies from conventional synthetic disease-modifying antirheumatic drugs, such as methotrexate, to biologic and targeted synthetic disease-modifying antirheumatic drugs, including TNF inhibitors, has reshaped expectations in clinical practice.1,2 Current recommendations emphasise early, remission-directed therapy; regular assessment with composite measures and patient-reported outcomes; and tapering and stopping the use of glucocorticoids.2,3 Within this evolving landscape, etanercept (a TNF receptor–Fc fusion protein) has an extensive, real-world track record that informs decisions about initiation, long-term use, and tapering for appropriate patients.4-6
In this expert discussion, Alten addresses key questions regarding what changed once TNF inhibitors became available to rheumatologists, how patients experience benefits in everyday life, how to apply structured safety screening and monitoring, and which patient profiles are well-suited to etanercept.
LOOKING BACK ON THE LAUNCH OF ETANERCEPT, WHAT DID IT MEAN FOR YOU AND PATIENTS?
“It was a paradigm shift,” Alten recalls. “In the late 1990s, many people with inflammatory arthritis were wheelchair-dependent in our clinics; today, the clinical picture is markedly different.” Etanercept was the first TNF inhibitor approved for RA,7 and its arrival offered patients an effective treatment and doctors an alternative treatment option to provide. Figure 1 provides an overview of therapies for RA.8
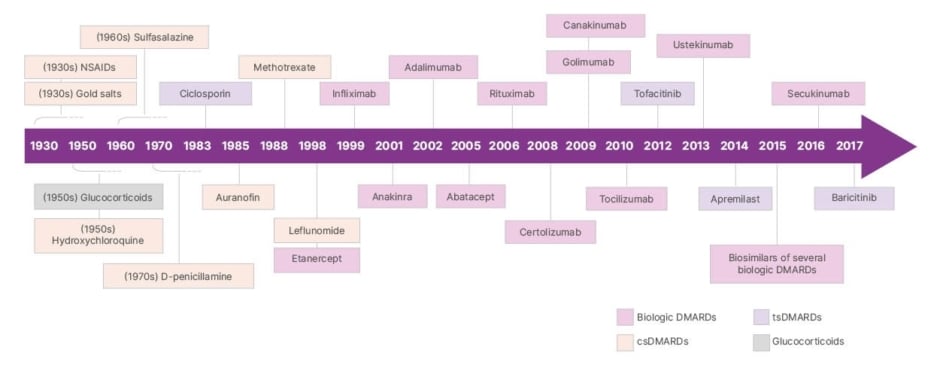
Figure 1: Evolution of therapy in rheumatology.
Adapted from Burmester et al.8
csDMARDs: conventional synthetic disease-modifying antirheumatic drugs; DMARDs: disease-modifying antirheumatic drugs; NSAIDs: non-steroidal anti-inflammatory drugs; tsDMARDs: targeted synthetic disease-modifying antirheumatic drugs.
“Patients often first feel the effects of the treatment as the lifting of pain that had become part of everyday life. Mornings are less painful, stiffness eases sooner, and stairs stop feeling like hurdles.” Patient-reported outcomes are vital in day-to-day care of patients with RA.9 “We still start every visit by asking about pain. With effective TNF inhibition, many patients report that their constant pain is fading. Function follows: our goal now is to preserve near-normal function.” Fatigue can also improve with etanercept and other TNF inhibitors, and it “deserves the same attention as pain and function.”10
IN THE LONG TERM, WHAT OTHER BENEFITS HAVE YOU OBSERVED, AND WHAT ARE THE SAFETY CONSIDERATIONS AND MONITORING REQUIREMENTS WITH ETANERCEPT?
Alten’s view is that, in the hands of experienced rheumatologists, etanercept has a well-established safety profile with a favourable benefit-risk balance. As she put it: “First of all, the monitoring in the hands of experienced rheumatologists makes [etanercept] a very well-tolerated drug, and so the benefit-risk profile is balanced, but it needs the experts.” She describes a practical safety framework that is visible to patients in primary care, including clear national society guidance, leaflets that support informed consent from the outset, materials for general practitioners to state which treatment a patient receives, and routine monitoring aligned with local policy.11 Over time, day-to-day experience has reinforced confidence in use. Concerns that were historically voiced about pregnancy have eased with accumulating literature and clinical experience. In practice, TNF inhibitors should only be used during pregnancy if the potential benefits to the mother outweigh the potential risks to the fetus.12-15
Etanercept is a fusion protein rather than a monoclonal antibody. This translates to fewer anti-drug antibodies compared with some other TNF inhibitors, no neutralising antibodies, and thus long-term persistence and adherence.14,16,17 According to Alten, the choice also extends to sensitive groups. “Early and sustained experience with anti-TNF therapy includes children, older adults, and patients with latent tuberculosis risk (with an appropriate testing and management pathway, such as initiation of prophylaxis of latent tuberculosis infection prior to therapy with etanercept).”15,18-20
IN WHICH PATIENT POPULATIONS DO YOU TEND TO INITIATE ETANERCEPT TREATMENT, AND WHY? ARE THERE ANY FOR WHOM IT IS NOT APPROPRIATE?
“First, I check for needle phobia. With auto-injectors, this is less of a problem, but if someone is firmly against injections, we can consider an oral small molecule. Infection risk is also important to consider before initiating a TNF inhibitor. During the COVID-19 pandemic, data from our German registry (led by Rebecca Hasseli) showed that continuing TNF inhibitor therapy with sensible precautions did not pose a greater risk to patients than stopping; high disease activity itself is the greater risk.21,22 Our aim is low disease activity or remission without steroids: treat-to-target.”
LOOKING AHEAD, WHAT ROLE DO YOU FORESEE ETANERCEPT PLAYING IN RHEUMATOLOGY TREATMENT PATHWAYS?
Alten emphasised the long-term durability of etanercept: “I think [etanercept] will keep its strong position. In Germany’s registry (Rheumatoide Arthritis: Beobachtung der Biologika-Therapie [RABBIT]), published annually, and across clinical trial programmes, including ESTHER, we see sustained effectiveness. I have patients who have taken [etanercept] continuously for more than 10–15 years, which I believe reflects the lower production of anti-drug antibodies because it is a fusion protein rather than a monoclonal antibody.”23
ARE THERE ANY ADDITIONAL INSIGHTS FROM CLINICAL PRACTICE YOU WOULD LIKE TO HIGHLIGHT?
“Many early-career rheumatologists have limited first-hand experience of the severe disease that was common before TNF inhibition. To keep that perspective alive, I maintain a curated ‘museum’ of historical images that we use in teaching to show what uncontrolled disease looked like. Operational pressures such as ward reorganisations and occasional unit closures can erode training opportunities. Protecting education is essential so that the specialty does not drift back toward pre-biologic outcomes.” Figure 2 illustrates the effect of treatment with etanercept for a patient with psoriatic arthritis.
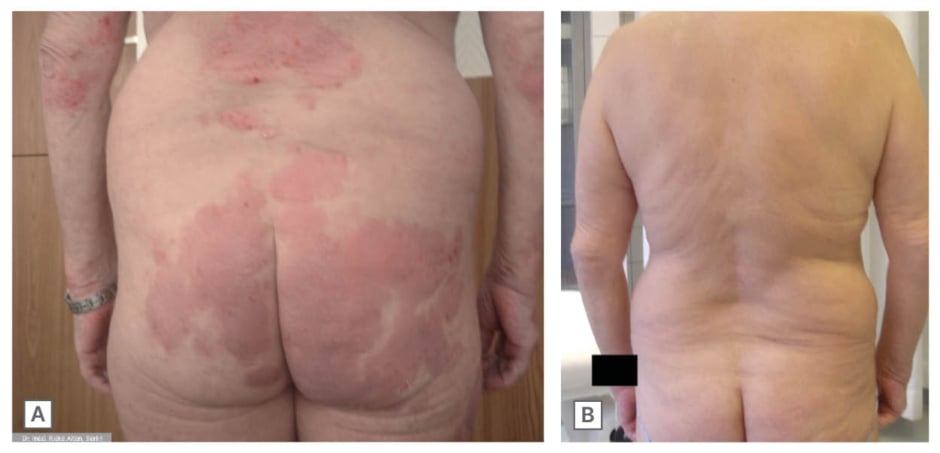
Figure 2: Psoriatic arthritis: skin clearance after etanercept treatment.
Extensive psoriatic plaques before treatment (A) and near complete clearance after initiation of etanercept (B).
Image courtesy of Rieke Alten.
WHAT IS THE MOST IMPORTANT TAKEAWAY FOR RHEUMATOLOGISTS?
Alten concluded: “I recommend tailoring the treatment to the individual’s specific needs and behaviours. Physicians need to get to know the patient beyond what is visible at first glance. It is vital to apply treat-to-target consistently, prioritise remission or low disease activity, and minimise the use of steroids.”
©2025 Pfizer Inc.
PP-ENB-GLB-0649
November 2025







