BACKGROUND AND AIMS
Patients with low-grade (LG) Ta non-muscle invasive bladder cancer (NMIBC) exhibit a high recurrence rate, while cancer-specific mortality remains notably low.1 Consequently, preserving patient quality of life through treatment deintensification emerges as a critical objective, simultaneously ensuring a minimal risk of progression to muscle-invasive bladder cancer.
In this context, active surveillance (AS) is increasingly recognised as a feasible management strategy for patients with small, presumably LG, recurrences of NMIBC.2,3 The International Bladder Cancer Group (IBCG) recently introduced a novel scoring system for intermediate-risk NMIBC.4 This study aims to assess the efficacy of the IBCG intermediate-risk (IR) scoring system in predicting the need for delayed transurethral resection of the bladder tumour among patients with LG NMIBC under active surveillance.
MATERIALS AND METHODS
The Bladder Italian Active Surveillance (BIAS) registry was utilised. The BIAS project is a prospectively maintained AS database of patients with a history of pathologically confirmed LG Ta/T1a NMIBC. The inclusion criteria for BIAS patient cohort include: LG papillary NMIBC, ≤5 apparent LG NMIBC at recurrence, tumour ≤1 cm in diameter, absence of gross haematuria, and no high-grade cancer cells on urine cytology. Subsequent transurethral resection of bladder tumour (TURBT) was offered to patients who no longer met the inclusion criteria or patient choice. The primary endpoint was the rate of delayed TURBT for AS events. Multivariable Cox proportional hazards analysis was used to determine factors associated with delayed TURBT following AS.
RESULTS
A total of 163 patients with LG Ta/T1 (208 AS events) were included for analysis. Delayed TURBT was performed in 109 patients (131 events), with a median follow-up of 24 months (interquartile range: 8–60 months). The median age was 71.6 years (interquartile range: 66.0–79.0), and 80.4% of the patients were male. Patients with no risk factors, one risk factor, and two or more risk factors accounted for 41 (20%), 120 (58%), and 47 (22%) AS events, respectively. Patients with no risk factors were three times more likely to continue AS compared to those with three or more risk factors at 24-month follow-up (61% versus 19%; Figure 1). A multivariable Cox regression model reported that the IBCG scoring system was associated with delayed TURBT (1–2 risk factors [hazard ratio: 1.8; 95% confidence interval: 1.06–3.06; p=0.030], three or more risk factors [hazard ratio: 3.76; 95% confidence interval: 2.07–6.81, p<0.001]), after adjusting for age and T stage. Grade progression occurred in 6% (n=10) of events, and stage progression developed in 3% (n=4) of events, in the cohort. Importantly, no patient developed NMIBC. The IBCG-IR scoring system was not associated with a higher risk of grade (p=0.9) or stage progression (p=0.2).
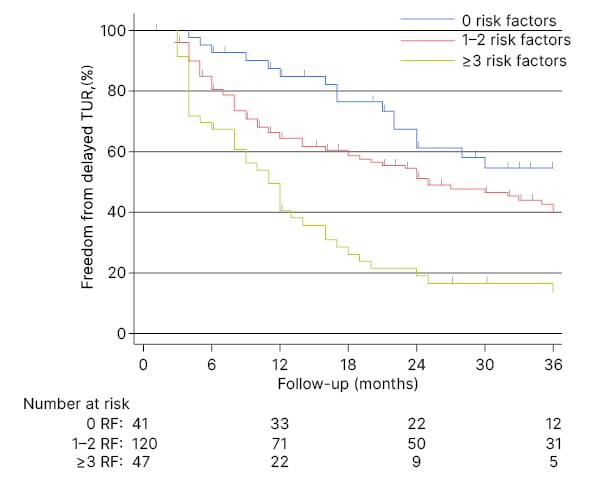
Figure 1: Kaplan–Meier analysis for freedom from delayed transurethral resection stratified by risk-factor groups according to active surveillance events (n=208).
RF: risk factor; TUR: test uncertainty ratio.
CONCLUSION
This collaborative research demonstrates that the IBCG IR-NMIBC scoring system effectively predicts the probability of sustained AS in patients from the BIAS study. Consequently, this scoring system facilitates enhanced patient counselling, and supports more informed collaborative decision-making between patients and physicians before initiating an AS regimen.







