Interview Summary
Hidradenitis suppurativa (HS) is a chronic, debilitating, inflammatory skin disease characterised by painful lesions that can cause disability and diminish patients’ health-related quality of life (HRQoL). Long-term disease control is therefore essential to prevent irreversible skin damage and disease progression. During interviews conducted by EMJ, three leading European dermatologists explored 3-year data from the Phase 3 BE HEARD I&II trials and the open-label extension (OLE) study of bimekizumab, a humanised monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F, indicated for the treatment of adult patients with moderate-to-severe HS. These data were presented recently in poster and oral presentations at the 2025 European Academy of Dermatology and Venereology (EADV) Congress and the Symposium on Hidradenitis Suppurativa Advances (SHSA).Thrasyvoulos Tzellos, Senior Consultant at Nordland Hospital Trust, Bodø; and Associate Professor at the University of Tromsø, Norway, highlighted the sustained disease control achieved with bimekizumab over a 3-year follow-up of the BE HEARD I&II studies, including improvements in stringent clinical endpoints. Antonio Martorell, Head of the HS Unit in the Dermatology Department at Hospital de Manises, Valencia, Spain, explored the concept of a window of opportunity in HS and the link between disease duration and outcomes in patients treated with bimekizumab. Finally, Sylke Schneider-Burrus from the Department of Skin Surgery, Havelklinik in Berlin, Germany, focused on key patient-reported outcomes from the BE HEARD I&II studies, including the positive impact of bimekizumab on skin pain and HRQoL.
BACKGROUND
HS is a chronic, painful, inflammatory skin disease that can progress if not effectively treated, causing irreversible damage and adverse sequelae.1-3 The hallmark symptoms of the condition are nodules, abscesses, and pus-discharging draining tunnels (DT), which typically affect the armpits, groin, and buttocks.1,2 People with HS experience flare-ups of the disease as well as severe pain, which can have a major impact on quality of life (QoL).1,2 HS typically develops in early adulthood and has an estimated global prevalence of around 1%.1,2,4
Bimekizumab is a selective inhibitor of IL-17A and IL-17F that is indicated for active, moderate-to-severe HS in adults with an inadequate response to conventional systemic HS therapy.5 IL-17A and IL-17F are two key cytokines that drive the underlying inflammatory processes in HS.5
BE HEARD I&II were multicentre, randomised, double-blind, placebo-controlled Phase III studies that evaluated bimekizumab in adult patients with moderate-to-severe HS.6 In each study, patients were randomised 2:2:2:1 to receive subcutaneous bimekizumab 320 mg every 2 weeks; bimekizumab 320 mg every 2 weeks to Week 16, then every 4 weeks to Week 48; bimekizumab 320 mg every 4 weeks to Week 48; or placebo to Week 16, then bimekizumab 320 mg every 2 weeks.6 Patients who completed Week 48 of bimekizumab treatment in the main trials could enrol into the OLE, which included on-label and off-label dosing arms. Of the total 1,014 patients, 556 randomised to bimekizumab at baseline in BE HEARD I&II completed Week 48 and entered the OLE, of which 367 completed Week 148 of treatment.7
BE HEARD I&II: BIMEKIZUMAB EFFICACY AND SAFETY PROFILE
Tzellos began by emphasising the value of long-term effective treatments for HS given the hallmark features of the disease. “HS is a chronic severe disease with long-term systemic inflammation…and there are specific features and characteristics of the disease that make it very challenging for patients,” he explained. “First of all, you have a disease that progresses from nodules and abscesses to fistulas. This extensive fistulation with fibrosis can destroy the skin and lead to scar formation.”
“We know that fistulation is a part of disease progression,” Tzellos continued, “so one part is fistulas and disease progression, the other is flares. It is important to have a treatment that can reduce the number of flares. Also, we have the need for deeper anti-inflammatory effects compared to previous treatments and the need for a long-term, well-tolerated treatment. So, these are the critical points when it comes to HS and future treatments as well.”
Focusing on results from BE HEARD I&II and the OLE study, Tzellos described how clinical improvements at Year 1 with bimekizumab were maintained or further improved through 3 years of treatment.7 At Week 48, Hidradenitis Suppurativa Clinical Response (HiSCR) 50, 75, 90, and 100 responses were 79.9%, 64.0%, 42.3%, and 30.2%, respectively.7 Notably, these responses were maintained to Week 148 with 90.2%, 81.2%, 64.3%, and 50.1% of patients achieving HiSCR50, 75, 90, and 100, respectively, after 3 years of treatment (all bimekizumab data quoted are observed cases, total OLE cohort, unless otherwise mentioned).7 “When it comes to all outcomes, it is clear that bimekizumab had a clinically meaningful benefit, which is not only stable through time, but also increasing,” noted Tzellos. “This means that continuing bimekizumab brings even more benefit in the long-term.”
Overall, bimekizumab was generally well-tolerated in the BE HEARD I&II studies and the OLE, and no new safety signals were identified with up to 3 years of treatment. The exposure-adjusted incidence rate for any treatment-emergent adverse event reported in up to 3 years of bimekizumab treatment was 226.8 patient-years, and actually showed a decrease with longer exposure to bimekizumab.7
“When it comes to safety, the 3-year data for bimekizumab are as expected according to the already known safety profile, so no new safety signals,” Tzellos confirmed. Oral candidiasis and upper respiratory tract infections are the most frequently reported adverse reactions with bimekizumab.5 “The Candida infection part is well known; it is manageable and can be treated,” Tzellos added.
Efficacy Across Lesion Types
As Tzellos outlined, bimekizumab demonstrated consistent effects across a range of HS lesion types in BE HEARD I&II and the OLE, which he described as “physical signs” of the disease. Clinically meaningful improvement in DTs, total tunnels, abscesses, and inflammatory nodules were seen at Year 1, with numerical increases to Year 3.8 After 3 years of bimekizumab treatment in BE HEARD, the proportion of patients achieving complete resolution across specific lesion types was: 83.5% (203/243) for abscesses (A100); 51.5% (189/367) for abscesses and nodules (AN100); 60.8% (202/332) for abscesses with DTs (A+DT100); 31.5% (103/327) for total tunnels (TT100); 62.9% (183/291) for DTs (DT100); and 54.8% (199/363) for inflammatory nodules (IN100).8
As Tzellos confirmed: “It is clear that we have benefit against nodules and abscesses. Previous drugs had such effects as well, but it seems that the effect is even higher (with bimekizumab).”
“But the most crucial part is the results we are seeing for fistulas,” he emphasised. “It is clear from the data we have for bimekizumab that there is a reduction, not only for the draining tunnels, but for the total number of tunnels as well. It is much more critical to show that a fistula has disappeared, rather than just moving from draining to non-draining status, as this shows not only prevention, but, to some extent, reversion of the fibrotic process.”
“Another important aspect of the bimekizumab data is not only reduction of areas involved, but [also] no new areas involved in the body: this is quite crucial as well,” Tzellos added.
Reduction in Draining Tunnels
DTs are pus-discharging tunnels that develop under the skin in patients with HS and result from long-term inflammation.9 These painful skin lesions are indicative of irreversible damage and require careful management. DTs have also been linked to increased low mood/depression, sleep disturbances, and fatigue when compared to patients with HS who have no tunnels.10,11
As Tzellos elaborated: “Draining tunnels are the most bothersome aspect of the disease when it comes to long-term pain, odour, and reduced quality of life. Also, it is the physical sign that will progress with fibrosis and scar tissue, so it is a clear point of further disease progression…and an unmet need.”
In the BE HEARD I&II studies, patients with moderate-to-severe HS treated with bimekizumab demonstrated a rapid reduction in DT count that was maintained over 3 years.12 At baseline, the mean DT count was 3.8.12 Mean absolute change from baseline in DTs was −2.4 at Year 1 and increased to −3.1 at Year 3.12 Overall, 48.2% (204/425) of patients achieved complete DT resolution at Year 1 of bimekizumab treatment, which further improved to 62.9% (183/291) by Year 3.8
Importantly, as Tzellos pointed out, this reduction in DTs and other major HS lesions with bimekizumab was seen regardless of patients’ baseline disease severity.8 “In the overall group, we see an achievement and positive effect on IHS4 100, draining tunnels, and draining tunnel 0, but we see this irrespective of the number of baseline draining tunnels, which is quite important,” he noted. These improvements are particularly noteworthy given the “baseline severity with the Phase III bimekizumab programme where more than 80% of patients had severe disease,” Tzellos added.6
Impact on International Hidradenitis Suppurativa Severity Score System
HiSCR has long been considered the gold standard primary outcome in HS clinical trials; however, the use of new and more stringent endpoints is increasingly gaining traction with the advent of more effective treatment options.13,14 One of these is the International Hidradenitis Suppurativa Severity Score 100 (IHS4 100), which evaluates the number of inflammatory nodules, abscesses, and DTs, and is defined as a 100% improvement from baseline. IHS4 100 is a particularly important clinical endpoint in HS as it equates to complete resolution of inflammatory lesions. As Tzellos expanded: “HiSCR does not include the reduction of draining tunnels and only relates to reduction of nodules and abscesses. On the other hand, effect on IHS4 score is much more important because it includes quantification of draining tunnels in a validated manner…Then you have a score that gives weight to the most bothersome physical sign of the disease, which is fistulas. If you have achievement in this score, then it is quite crucial because you have clear evidence that this treatment is effective against all aspects of the disease.”
Focusing on IHS4 100 results from the BE HEARD I&II studies, Tzellos outlined how 12.5% of patients treated with bimekizumab achieved IHS4 100 at Week 16, increasing to 25.2% at Week 48, and 40.1% by Year 3 (Figure 1).8 “Practically this means not only complete inflammation resolution, but disease remission,” he emphasised.
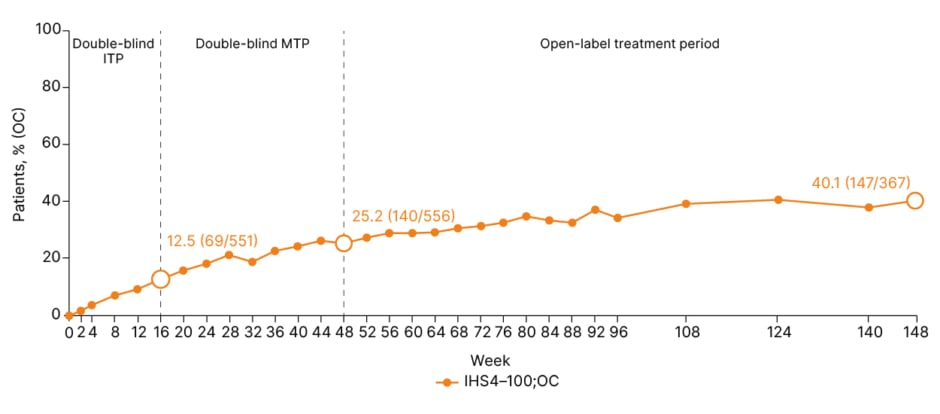
Figure 1: Proportion of patients achieving International Hidradenitis Suppurativa Severity Score 100 over 3 years.8
OC: n/N: denominator represents number of patients with a non-missing lesion count assessment in the given week, and percentages are calculated accordingly. The requirement of a visit at Week 48 to enter the OLE resulted in an increase in N number at Week 48. IHS4-100 mNRI at Week 16/48/148: 12.5%/25.2%/33.2%. IHS4: International Hidradenitis Suppurativa Severity Score; ITP: initial treatment phase; MTP: maintenance treatment phase; mNRI: modified non-responder imputation; OC: observed case; OLE: open-label extension.
“If you asked someone 2 or 3 years ago, can we have complete resolution of all inflammatory lesions, you would probably hear no because this is not easily achieved,” Tzellos continued. “And why? Because IHS4 100 means that all inflammatory lesions, nodules, abscesses, and draining fistulas are zero.”
Clinical evidence suggests that achieving high efficacy thresholds in HS, as represented by stringent clinical endpoints like IHS4 100, results in less pain and improved QoL for patients.15
BE HEARD Clinical Data in Context
Putting the 3-year results from the BE HEARD I&II trials into context, Tzellos observed that: “When we started treating HS with biologics, the interest was in reducing inflammation. That’s why the first scores, like HiSCR, were more concentrated to nodules and abscesses. And it was a big step that we managed to reduce nodules and abscesses, but it is an even bigger step to be able to show a reduction or a reversal of progression.”
“When you have a consistent 3-year, long-term effect, not only reducing but maintaining areas without new lesions in a stable manner, this is quite crucial, and suggests bimekizumab has the potential to reverse progression to some extent,” he concluded.
A WINDOW OF OPPORTUNITY IN HIDRADENITIS SUPPURATIVA
Delays in initiating treatment have been linked to reduced clinical response in HS, highlighting the existence of a ‘window of opportunity’ for effective intervention.14,16 Martorell explained that seizing this window of opportunity involves starting treatment when lesions are still reversible and “medical therapy can achieve the best results,” in other words, when a patient has “nodules, abscesses, or early tunnels, but no fibrosing structures.” Once DTs are formed and undergo epithelialisation, they do not respond to medical treatment, leaving surgery as the only management option.14 The existence of this window of opportunity in HS is now confirmed by the clinical data, Martorell explained, and European experts in HS have recently developed a consensus definition to aid in its clinical application (Figure 2).14,17
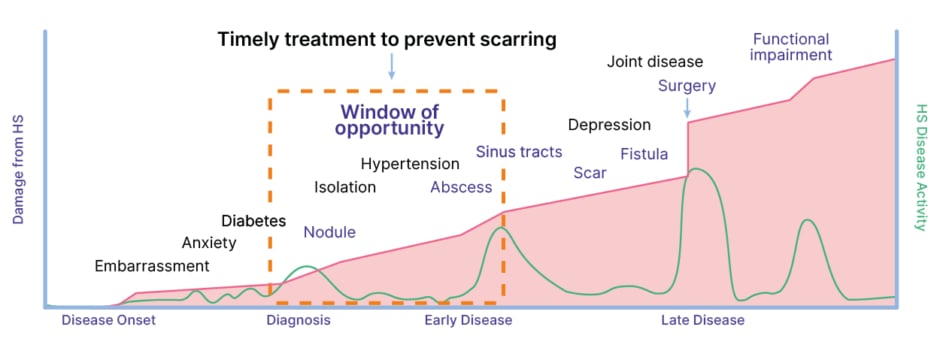
Figure 2: The window of opportunity in hidradenitis suppurativa.17
HS: hidradenitis suppurativa.
Martorell outlined how HS advances through three different Hurley stages, categorised by the presence and extent of lesions, scarring, and sinus tracts, and underscored how early intervention is crucial to slow or prevent this progression from mild to severe disease.18 “If we don’t treat our patients with effective drugs that stop the development of abscesses and tunnels, we are not going to be able to stop this progression of the disease. We are not going to be able to stop the situation in which patients will need complex surgeries, like plastic surgeries or general surgeries,” he stressed.
Martorell went on to describe how disease progression can be defined clinically in HS as the development of at least one new tunnel and/or the extension in size of existing tunnels. “We can also consider progression in patients that are developing at least one new persistent HS lesion in an anatomic area that was not previously affected. And last, but not least, we consider that any increase in the number of persistent HS lesions in previously affected anatomic variations can be also considered progression of the disease,” he confirmed.
Disease Duration and Outcomes in Bimekizumab-Treated Patients
“Delay in diagnosis is impacting results we can achieve with the drugs we have now available in clinical practice,” stressed Martorell. In illustration of this point, he outlined results from a subanalysis of the BE HEARD I&II data that considered the impact of disease duration on IHS4 outcomes in bimekizumab-treated patients. Subjects were separated into groups according to lowest (<2.38 years) and highest (≥10.74 years) disease durations.19 Focusing on results for IHS4-75, Martorell noted that 61% and 74% of patients in the shorter disease duration group achieved this outcome at Weeks 46 and 96, respectively, compared to 53.4% and 62.4% in the longer duration group. Improved outcomes in the shorter disease duration group were also seen across other IHS4 endpoints and DT counts, particularly at the higher efficacy thresholds.19 As Martorell explained, these results highlight the potential impact of early treatment with bimekizumab in patients with HS in order to maximise clinical outcomes. The importance of not missing this optimal window of opportunity for biologic intervention in HS was echoed in a recent questionnaire-based study of 55 HS specialists. In this survey, many clinicians felt that delays in starting effective treatment can lead to Hurley progression and the development of irreversible lesions.16
Martorell also reiterated the importance of demonstrating benefit against dynamic and stringent clinical endpoints like IHS4 100, which he said would have been considered “impossible” until recently. Referencing the BE HEARD I&II data, Martorell emphasised that “four out of 10 patients achieved IHS4 100 in Year 3 of follow-up.”
“This is relevant, not only for complete control,” he continued, “but the effectiveness of the drug on each elemental lesion, because we know the turning point of HS is the tunnel. And when we check a subanalysis of this IHS4 100, we can see how up to 62.9% (183/291) of patients can achieve complete control of DTs after 3 years.”8
Optimising Therapeutic Position
Martorell suggested that bimekizumab could be “the ideal drug to treat patients in this window of opportunity in HS” given the presented data from BE HEARD I&II showing better outcomes with shorter disease duration and control of a high percentage of DTs at 3-year follow-up.12,19 “If we consider the draining tunnel as the structure that is defining this disease progression,” he noted.
“Regarding the mechanism of action of bimekizumab, the dual blockade of IL-17A and IL-17F may explain its efficacy across different stages of the disease, particularly within the window of opportunity, where treatment response is closely linked to the type of tunnels present in each patient,” Martorell explained. He further proposed that HS tunnels can be classified into four stages based on the progressive structural characteristics of these lesions over time.20
“The dermal tunnel (Type A) represents the earliest stage of this process. It originates within the dermis and remains confined to this layer, without epithelial lining,” Martorell noted. “The next step in tunnel progression is the development of the dermoepidermal tunnel (Type B), in which the dermal structure comes into contact with the epidermis, often leading to active drainage. This direct dermoepidermal interaction promotes progressive epithelialisation of the tunnel walls over time, which markedly reduces the ability of medical treatments to achieve complete disease control. If tunnels are not treated at this stage, they may evolve into complex tunnel formations (Type C), characterised by multiple interconnected tracts, fibrotic tissue, and network-like structures, which clinically correspond to Hurley Stage III disease,” he pointed out.
“In addition, particular attention should be paid to dermal tunnels located in gluteal or perineal areas,” Martorell continued. “In these anatomical regions, especially in patients who spend most of the day in a seated position, dermal tunnels are often unable to drain through the epidermis. As a consequence, the inflammatory tract tends to redirect its drainage through the subcutaneous tissue, progressively dissecting deeper planes along fascial structures. This process may allow the tunnel to extend beyond its original location, in some cases opening into distant anatomical areas such as the inguinal region. In this scenario, the lesion should be considered a subcutaneous tunnel (Type D), representing a distinct and more aggressive pattern of tunnel progression.”
Martorell went on to describe how bimekizumab could be acting against these four different stages of tunnels in HS. “Bimekizumab is acting in the early stages, in order to control tunnel development and prevent progression, but also in the late stages in order to prevent the increase in size of structures that probably will need a complex surgery in order to achieve internal control of the disease,” he postulated.
Asked about the optimal therapeutic positioning of bimekizumab in the current HS treatment landscape, Martorell suggested that data from BE HEARD I&II could potentially provide a stimulus for earlier treatment. “When you see the 3-year data, the persistence of effectiveness during these 3 years and even an increase in the response, and specifically what we can achieve if we treat patients in the early stage of the disease, I think, in my opinion, that we need to consider bimekizumab as the first option in order to stop the progression of the disease,” he remarked. Specifically, Martorell indicated that the use of bimekizumab in the initial stages of HS may have the potential to improve QoL for patients by reducing or stopping flares, as well as preventing the development of new tunnels and controlling DTs. “We have data that confirm that the drug can work even better if we use it in this first stage of the disease,” he reiterated.
This point was also echoed by Tzellos: “It seems that, especially when it comes to achievement of IHS4 100, complete resolution of inflammation, […] it is better to treat early in the disease. So, trying many agents and leaving bimekizumab as the last does not make sense.”
PATIENT PERSPECTIVES
Moving on to consider patient perspectives and key patient-reported outcomes in HS, Schneider-Burrus stressed that: “First of all, it is important to state that there is no other dermatologic disease that has the same impact on quality of life as HS.”
“Studies conducted around the world have confirmed that if you compare the impact of dermatologic diseases on quality of life, then HS is always the worst,” she elaborated, “and one factor that has a stark correlation to the impacted quality of life is pain.”
A recent prospective longitudinal study presented at SHSA 2025 found that HS flares significantly impaired both mental health and QoL in patients with HS. Referencing this study, Schneider-Burrus noted: “There is a very strong correlation between flares and pain and quality of life…which also gives us an option to help patients with their quality of life if we get flares under control.” In this study, 93.7% of patients showed increased HRQoL impact during flares.21 Pain and the number of skin lesions were the main indicators of a disease flare-up.21
Schneider-Burrus explained how disease flares and pain can majorly impair patients’ day-to-day lives by affecting their ability to go to work and care for their family. “This is not just something that we can measure, but in real life, it is something that patients deal with every day,” she emphasised.
Attaining full control of HS symptoms is a key element in improving overall patient HRQoL, although Schneider-Burrus noted that patients’ personal treatment goals can be very individualised. “For the average patient, they don’t want to have skin that’s scar-free, but they want to be pain-free, and they want to be able to take part in their regular life. They want to be able to go to work, to be able to be with their family and do normal day-to-day […] activities,” she explained.
Schneider-Burrus also highlighted the unpredictability of HS flares that “can sometimes come out of nowhere, and that is very impacting for patients because they never know if they can count on their body.” She continued: “The unreliability of their bodies, from when I talk to patients, that’s one of their major issues…they want to be pain-free, no draining pus, no impact on their QoL, and to be able to plan their future.”
As Schneider-Burrus explained, pus and oozing from HS lesions, along with the associated smell, can collectively impose a substantial burden on patients with HS and their caregivers. Lesions can prove difficult and time-consuming to manage and impose restrictions on patients’ clothing choice. “One thing is the oozing and the time consumption, but also the dislike of their own body,” she pointed out. “Patients develop a disgust [towards] their own body and can’t accept it.”
Patient-Reported Outcomes in the BE HEARD I&II Trials
QoL in bimekizumab-treated patients was evaluated in the BE HEARD I&II studies using the Dermatology Life Quality Index (DLQI). “A DLQI score of 0 or 1 means no impact on QoL, which is basically equivalent to somebody who has no dermatologic disease,” Schneider-Burrus explained. “Usually, patients with HS have a high DLQI score, and if you look at the BE HEARD study data, the average of the baseline DLQI was around 11.”11
In the BE HEARD I&II studies, bimekizumab produced improvements in QoL at Year 1 that were maintained or further improved through 3 years of treatment.11 “After 1 year, 27.4% had a DLQI of 0 or 1, that means a quarter of patients basically have a normalised life,” noted Schneider-Burrus.
“You see even an improvement after that, in the open-label treatment period,” she continued. “After 3 years of treatment, 38.1% of patients had a DLQI of 0 or 1. They might still have something showing on their skin, like dried-out areas or scars, but it’s not impacting their life, and that’s something new that we hadn’t seen in other treatments before.”
Schneider-Burrus explained that patients’ typical experience with previous treatment approaches in HS was an initial improvement, followed by a worsening. “So, to have a treatment that has not just a stable response in items we can count but also in patient outcomes is amazing,” Schneider-Burrus concluded, adding that these positive results also provide impetus for patients to try new biologic treatment approaches such as bimekizumab.
Skin Pain and Mental Health
Skin pain is experienced by the majority of patients with HS and is widely acknowledged to be one of the most debilitating symptoms of the disease.22 Skin pain outcomes in patients receiving bimekizumab in BE HEARD I&II were evaluated using the HS Symptom Questionnaire (HSSQ) skin pain item, which assesses patients’ perceptions of HS skin pain over the past 7 days using an 11-point numeric rating scale with ‘0’ indicating “no pain” and ‘10’ indicating pain “as bad as you can imagine.”23 As Schneider-Burrus outlined: “The overall mean absolute HSSQ score is reduced quite impressively with treatment. After 1 year, you see almost a 50% [48.0%] reduction from a total of 5.8 down to 2.8. Then, over time, the skin pain is reduced even more … it shows that the treatment is effective over a long period. After 2 years, it is reduced to 57.1% less than baseline, and after 3 years, to 62.4% less than baseline.”23 (Figure 3)
She continued: “The other way is to look at the proportion of patients that are in different skin pain categories. At baseline, more than 50% have severe or very severe pain, and this shifts to mainly no skin pain or mild skin pain. The group of patients who suffer from severe or very severe pain is only 11% after 3 years. So, there is still a group of patients who suffer from pain, but it is reduced to less than a fifth of baseline.”23
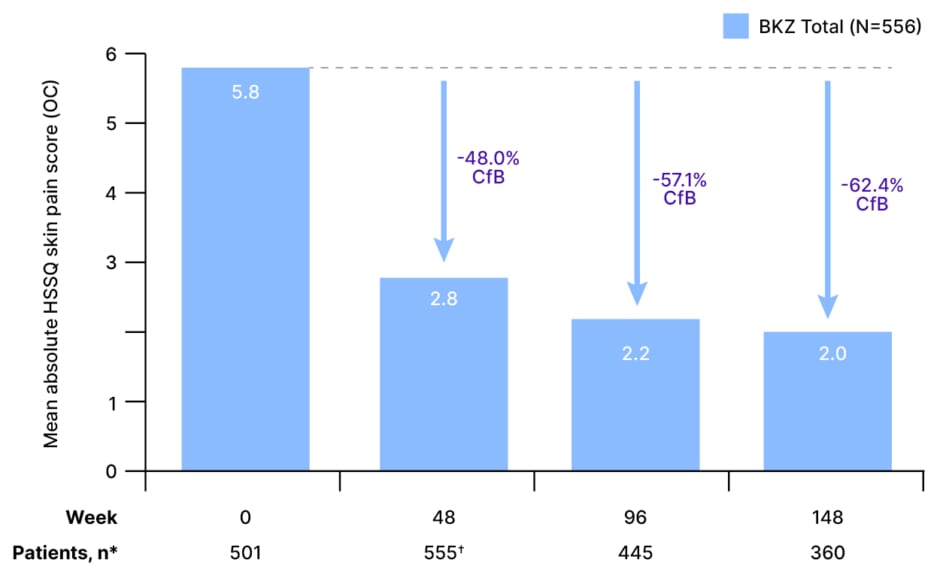
Figure 3: Mean absolute and percentage change from baseline in Hidradenitis Suppurativa Symptom Questionnaire skin pain scores over 3 years.23
*For OC, n numbers are reported for mean absolute HSSQ skin pain score in the given week. Mean percentage CfB in HSSQ skin pain scores, n: Week 48, 543; Week 96, 436; Week 148, 354.
?The requirement of a visit at Week 48 to enter the OLE resulted in an increase in n number at Week 48.
BKZ: bimekizumab; CfB: change from baseline; HSSQ: Hidradenitis Suppurativa Symptom Questionnaire; OC: observed cases; OLE: open-label extension.
As Schneider-Burrus explained, depression and anxiety occur frequently in patients with HS and are tightly connected to pain and overall QoL. Mental health was evaluated in the BE HEARD I&II trials and, after 2 years of bimekizumab treatment, the proportion of patients without depression increased from 74.0% at baseline to 89.8% at Week 96.24 “That’s really important for patients, because depression is one of the most common and important comorbidities,” she stressed.
“So, we don’t just have an impact on the skin itself and on QoL,” continued Schneider-Burrus, “but depression, as a single item, also decreases in frequency, as does anxiety.”24
CLINICAL PRACTICE IMPLICATIONS
In conclusion, experts underscored the value of recently presented data from the BE HEARD I&II trials and the OLE, which have confirmed the depth and durability of response to bimekizumab over long-term follow-up, with clinical improvements maintained over 3 years of treatment.
Martorell explained that results showing that the initial rapid response to bimekizumab is maintained at 3 years have helped to contextualise the value of this biologic within the HS treatment landscape. Referencing, in particular, the progressive numerical improvement in HiSCR 50/75/90/100 responses from Week 48 to Week 148, Martorell suggested that: “This picture of the short- and long-term results we can achieve with bimekizumab confirms that we are in front of a drug that is going to ‘revolutionise’ the management of patients with HS.” He noted that disease stabilisation attained with bimekizumab is additionally relevant due to its impact on pain, psychosocial aspects of the disease, and patient QoL. “The data shown here represent control, not only of disease progression, but also control of the different symptoms that are going to limit the life of our patients,” he remarked.
Schneider-Burrus agreed that the BE HEARD 3-year bimekizumab data are clinically relevant and may help reinforce the value of long-term treatment for patients with HS. “For other treatments, we often saw that the disease worsens again after a while. So, to show data that support improvements, not just within the first weeks or months, but over a long time, […] is really exciting.” She explained that clinical practice is already changing to reflect these improved outcomes, with patients starting bimekizumab no longer being routinely scheduled for future surgery at the same time as systemic treatment initiation. “This is something new that we have not experienced with other treatments before,” Schneider-Burrus concluded. “It is not just the physical and measurable aspects, but it also creates a lot of hope for patients.”
As Tzellos concluded: “We have 3-year data showing benefit in all physical signs, not only inflammatory but non-inflammatory as well, and achievement of important physician-reported outcomes and patient-reported outcomes. So, in my opinion, both as an expert in the evidence but also as a clinician treating HS cases for more than 10 years, it seems that bimekizumab is the most effective treatment we have up to now.”
GL-BK-2500311 | Date of preparation: January 2026
© UCB Biopharma SRL, 2026. All rights reserved.