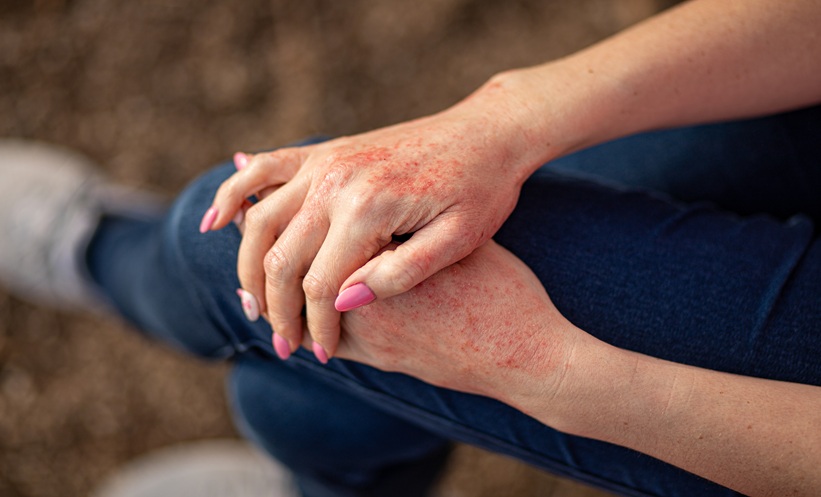
A NOVEL topical Janus kinase (JAK) inhibitor, pumecitinib 3% gel, has demonstrated strong efficacy and a favourable safety profile in adults with mild-to-moderate atopic dermatitis (AD), according to results from a multicentre phase IIb clinical trial.
The randomised, double-blind, placebo-controlled study evaluated pumecitinib 3% gel (PG-011), a selective JAK1/2 inhibitor, applied either once or twice daily over an 8-week treatment period. A total of 139 participants with mild-to-moderate AD were enrolled and randomly assigned in a 1:1:1 ratio to receive twice-daily pumecitinib, once-daily pumecitinib, or placebo.
Twice-Daily Pumecitinib Demonstrates Superior Efficacy
The primary endpoint was the percentage change in Eczema Area and Severity Index (EASI) score from baseline to Week 8. Results showed a marked improvement in patients treated with pumecitinib, particularly with the twice-daily regimen. At Week 8, mean EASI score reductions were -83.6% in the twice-daily group, -44.0% in the once-daily group, and -22.0% in the placebo group. Both active treatment arms achieved statistically significant improvements compared with placebo (P < 0.006), with twice-daily application demonstrating superior efficacy to once-daily use (P < 0.001).
Secondary efficacy outcomes further supported these findings. Higher proportions of patients receiving pumecitinib achieved Investigator’s Global Assessment scores of 0 or 1, as well as EASI 50, EASI 75, and EASI 90 responses, compared with placebo. Improvements in quality of life were also observed, with the twice-daily regimen consistently outperforming once-daily treatment.
Importantly, pumecitinib 3% gel was well tolerated. The incidence of adverse events was identical in the pumecitinib and placebo groups (48%), and no new safety concerns were identified. Local tolerability was favourable, and systemic exposure remained low, with mean plasma drug concentrations ranging from 38–104 pg/mL throughout the study.
Implications for Future Topical Therapies in Atopic Dermatitis
The authors conclude that pumecitinib 3% gel offers an effective and safe topical treatment option for adults with mild-to-moderate AD. The superior efficacy of twice-daily dosing suggests an optimal regimen, while low systemic absorption supports its potential as a targeted topical JAK inhibitor. These findings position pumecitinib as a promising addition to the evolving therapeutic landscape for atopic dermatitis.
Reference
Zhang Li et al. Efficacy and safety of pumecitinib 3% gel in treating mild-to-moderate atopic dermatitis: a multicentre randomized double-blind parallel placebo-controlled phase IIb clinical trial. Br J Dermatol. 2026; 194(2):236–243.