INTRODUCTION
Food allergies affect up to 10% of the population in the USA and have been increasing in prevalence over the last 2–3 decades.1 These allergies typically present with a reproducible, specific set of symptoms that occur immediately after ingesting a food. In this article, the authors discuss the approach to diagnosing food allergy, which centres on a thorough clinical history, followed by skin, blood, and/or oral testing if indicated. The authors also review current management options for food allergy, which are likely to expand in the future, given the ongoing research focus on this important public health concern, with several new treatments currently under investigation.
DIAGNOSIS
Clinical History
The clinical history is the most critical ‘test’ for diagnosing a food allergy. For most patients, symptoms of IgE-mediated food allergy occur within minutes to 1–2 hours after exposure.1-3 The following elements of the clinical history should be obtained: (1) the food causing the symptoms and its preparation (cooked or uncooked); (2) the specific symptoms experienced; (3) the timing of symptom onset in relation to ingestion; (4) the length of the reaction; (5) contributing factors, such as viral illness or medication use; (6) treatments given to stop the reaction; and (7) the reproducibility of the reaction with subsequent exposure. The clinician should also assess for other forms of atopy, such as atopic dermatitis, asthma, or allergic rhinitis (Table 1).2,4
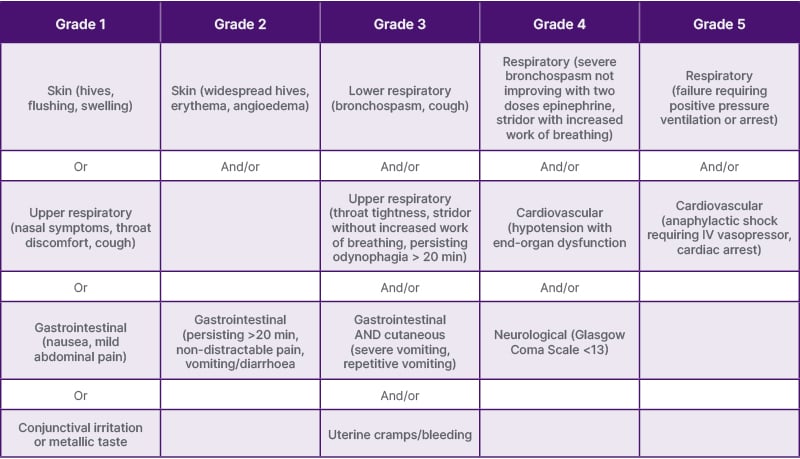
Table 1: World Allergy Organization grading scale for systemic allergic reactions
Adapted from World Allergy Organization Anaphylaxis Committee and Allergen Immunotherapy Committee 2024 Joint Statement.5
Grade 3–5 reactions are considered anaphylaxis by World Allergy Organization Anaphylaxis definition.
Signs and Symptoms
The typical symptoms of an IgE-mediated allergic reaction include:
- Skin manifestations (erythematous rashes, urticaria, angioedema)
- Respiratory symptoms (rhinorrhoea, nasal itching, wheezing, stridor)
- Gastrointestinal symptoms (vomiting, diarrhoea, abdominal pain)
More severe manifestations affect the cardiovascular or nervous systems, and may cause dizziness, tachycardia, or altered mental status. The most serious allergic reaction is anaphylaxis, a severe, multi-organ response that may potentially progress to shock and death, if not immediately treated.2,4
Diagnostic Testing
Once the clinical history is collected, diagnostic testing is the next step to confirm the culprit allergens. The gold standard for diagnosing food allergy is the oral food challenge (OFC), performed under medical supervision. During this test, the allergist administers escalating doses of the suspected food, monitoring carefully for any signs of an IgE-mediated reaction. OFCs are considered positive when objective symptoms are observed. While subjective symptoms may indicate a positive reaction, especially if worsening, repeated, or persistent, the decision to stop the challenge ideally should be based on the occurrence of objective signs of an allergic reaction.6,7
Skin prick tests (SPT) or food-specific IgE (sIgE) blood tests are commonly used initially in clinical practice. However, these tests should only be performed in combination with a detailed clinical history suggestive of IgE-mediated food allergy, as false positives are common. Both SPTs and sIgE testing have a sensitivity >90%, but a specificity of <50%. A negative result has a high negative predictive value, indicating a low risk of proceeding with an oral challenge to confirm the absence of the allergy. With a positive result, the size of the wheal formed during SPT, or the magnitude of sIgE, correlates with the likelihood of reactivity, but not the severity of a potential reaction. These test results can thus help the allergist and patient/family to better understand the likelihood of reaction if an OFC is performed. However, due to the low specificity of sIgE and SPTs, positive findings alone cannot definitively confirm the presence of food allergy. If skin and blood testing is negative, but the food has been avoided in the diet, or if test results are conflicting, an OFC can be helpful.4
TREATMENT
Current treatment options for food allergy include avoidance, oral immunotherapy (OIT), and omalizumab, with several promising new therapies currently in development. Given the risks, benefits, and logistical challenges associated with each treatment, it is critical that allergists engage in shared decision-making with the patients and their families to ensure that the treatment plan aligns with their individual goals and lifestyles.
Avoidance
Management for most patients with food allergy involves strict avoidance of allergens and treatment for acute reactions. Patients and their families should be counselled, ideally by a registered dietician, about strategies to avoid specific allergens. It is important for clinicians to provide practical guidance on allergen avoidance, including how to read food labels, strategies for dining out, and age-appropriate education that begins in childhood and continues into adulthood.4 Since there always exists a risk of accidental ingestion, patients should also carry an epinephrine autoinjector pack (2 doses) at all times, as this can be a lifesaving therapy in the case of an anaphylactic food reaction. Barriers to this approach, unfortunately, include the high cost of epinephrine autoinjectors, inadequate reimbursement by some insurance plans, and global shortages due to production issues.
Oral Immunotherapy
Historically, the only option for managing food allergy was strict allergen avoidance. However, the use of OIT has been becoming increasingly recognised as a method to attenuate allergic responses over time. OIT is time- and labour-intensive, carries a risk of adverse reactions, and its effectiveness as a definitive cure remains uncertain. Of note, in the USA, there is only one FDA-approved product for peanut OIT called Palforzia® (Aimmune Therapeutics, Brisbane, California, USA). However, OIT for other food allergens is being performed by some allergists using products that do not carry FDA approval.6-8
Treatment course
When initiating OIT, patients first undergo a supervised rapid dose-escalation phase in the clinic to demonstrate the safety and tolerability of doses of the allergen, prior to starting at-home dosing at a lower dose level. Then, during the build-up phase, this amount of daily home dose is increased every few weeks during up-dosing clinic visits until a target maintenance dose is reached. The last phase is the maintenance phase, during which home OIT daily dosing continues to maintain desensitisation. This phase typically lasts 3–5 years, as immune changes typically plateau after this period. Some patients prefer to continue dosing indefinitely for protection, while others opt to discontinue daily dosing and perform an OFC to assess for remission while off therapy.9
Benefits of oral immunotherapy
Several studies have demonstrated that, while desensitised, patients with food allergy are protected against accidental exposures to amounts below their reactivity threshold. Some have been able to include substantial amounts of the allergen in their diet. Protection against even small amounts has been shown to improve anxiety and quality of life significantly. Through the IMPACT trial, OIT has also been shown to increase the chance of achieving remission, defined as an absence of symptoms upon exposure to the allergen for 26 weeks after discontinuation of OIT.9,10
Key considerations before starting oral immunotherapy
Because OIT requires a significant commitment of time and effort, it is important to carefully evaluate whetherit is the right option for each patient.
Before starting OIT, comorbidities such as asthma, allergic rhinitis, allergic dermatitis, and chronic sinusitis must be well controlled. Additionally, it is important to evaluate the patient’s anxiety and determine whether any interventions are needed in advance. The caregiver’s relationship with the patient, as well as any family logistics that may affect adherence to treatment, should also be addressed.11 Moreover, patients with very elevated IgE levels or body weight may not be eligible for treatment.12
Factors to consider for patients who may benefit from OIT include the likelihood of not outgrowing their food allergy, the impact of the allergy on their quality of life, a history of anaphylaxis, and the patient’s individual goals, such as the desire to tolerate larger amounts of the allergen. However, risks may include reactions ranging from mild-(e.g., rash, oral itching) to-severe/fatal (e.g., anaphylaxis), as well as the development of eosinophilic gastrointestinal disorders.
Therefore, especially in children, dosing fatigue, food aversion, and anxiety may affect treatment adherence.11
Omalizumab
Based on data from the landmark Phase 3 clinical trial, Omalizumab as Monotherapy and as Adjunct Therapy in Children and Adults (OUtMATCH), omalizumab is the only FDA-approved treatment to reduce allergic reactions following incidental exposure to food allergens. It binds to the FcεR1 epitope of human IgE, thereby interfering with the binding of IgE to the IgE receptor. Over time, this results in the inhibition of mast cell and basophil activation upon allergen exposure.13
Benefits of omalizumab
Omalizumab offers the advantage of treating several allergic comorbidities along with food allergy. Furthermore, it can treat multiple food allergies by targeting free IgE. Adverse reactions usually involve injection site reactions, with anaphylaxis in rare cases. However, compared to OIT, omalizumab offers a less intensive monitoring regimen, with self-administration at home a potential option based on careful assessment of risk for anaphylaxis. Therefore, omalizumab may be better suited for patients with a difficult-to-control asthma, multiple food allergies, or those who prefer less frequently dosed therapy.14
Key considerations before starting omalizumab
Despite the availability of an FDA-approved dosing table, the ideal dose and dosing schedule are still not well understood. Additionally, patients with very high total IgE levels or body weight would fall outside of the dosing table parameters for omalizumab. There are no studies that can guide when omalizumab should be initiated, it can be started at any stage of having a food allergy, whether recently diagnosed or having lived with the allergy for years.14 It is also important to consider that some patients will not experience a significant clinical response to omalizumab for food allergy. In the OUtMatch study, only 67% of patients achieved the primary endpoint of consuming one dose of 600 mg of peanut protein without any significant symptoms.13
CONCLUSION
The diagnosis of food allergy poses a clinical challenge. Obtaining a clinical history consistent with an IgE-mediated food reaction is essential before pursuing SPT or sIgE blood testing, since these tests have low specificity, and a positive result does not necessarily indicate that a reaction will occur. Negative testing, however, has a high negative predictive value. Oral food challenges are utilised to attempt to clarify tolerance versus reactivity, typically when initial skin or blood testing indicates low risk of reaction.
Although strict avoidance has traditionally been the main management strategy for food allergy, and remains so at this time, there have been significant recent advances in developing novel treatments to achieve desensitisation and remission. OIT has gained more recognition as a potential method for decreasing the risk of severe life-threatening accidental food reactions if the amount ingested is below the reactivity threshold. However, it is a time-intensive process and potentially cost-prohibitive for some patients. OIT does carry potential risks of adverse reactions, and while it often does not enable patients to fully incorporate the food into their diet, it can often allow them to consume partial amounts without significant reactions. There is now an FDA-approved OIT for peanut allergy, which is likely to expand the use of this treatment option to more health systems and clinical sites. Even more recently, although significantly more expensive, omalizumab has been approved by the FDA for use in food allergy to prevent reactions to accidental ingestions. Further study is needed to understand the ideal dosing and the practical use of this treatment option.





