Meeting Summary
This article is based on presentations of a late breaking clinical trial at the European Society of Cardiology (ESC) Congress in conjunction with the World Congress of Cardiology (WCC), which took place between 29th August–1st September 2025 in Madrid, Spain. The presentations described analyses of MAPLE-HCM, a Phase III, head-to-head, comparative efficacy and safety study evaluating aficamten versus metoprolol as monotherapy in adults with symptomatic obstructive hypertrophic cardiomyopathy (oHCM). Pablo Garcia-Pavia, Hospital Universitario Puerta de Hierro, Madrid; and Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain, presented the primary and secondary outcomes of MAPLE-HCM, showing that 24 weeks of aficamten led to significant and clinically meaningful improvements in exercise capacity, symptoms, left ventricular outflow tract (LVOT) gradient after the Valsalva manoeuvre, N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, and structural cardiac remodelling. By contrast, metoprolol led to a reduction in the exercise capacity of these patients and, despite demonstrating on-target haemodynamic effects, failed to reduce LVOT gradients either at rest or following the Valsalva manoeuvre. Sheila Hegde, University of Texas, Southwestern Medical Centre, Dallas, USA; and Brigham and Women’s Hospital, Boston, Massachusetts, USA, presented further substantial improvements in multiple measures of cardiac structure and function with aficamten versus metoprolol. Overall, MAPLE-HCM data highlight the superior efficacy of aficamten over metoprolol, while maintaining an acceptable safety profile. These results strengthen the positioning of aficamten as monotherapy or potential first-line therapy for patients with symptomatic oHCM.
Background
oHCM is characterised by a hypercontractile left ventricle, myocardial hypertrophy, and LVOT obstruction, resulting in limiting symptoms and reduced exercise capacity.1,2
Aficamten, an investigational, next-in-class cardiac myosin inhibitor, has been developed to target the underlying pathophysiology of oHCM by reducing myocardial hypercontractility.2,3 Clinical studies have demonstrated that, when administered to patients with symptomatic oHCM despite standard-of-care therapy, aficamten has consistently been associated with an improvement in exercise capacity,2,3 a reduction in symptom burden,4 a normalisation of LVOT gradients,3,5 a decreased eligibility for septal reduction therapy,3 improvements in cardiac biomarkers,6 and favourable effects on cardiac structure and function.5,7
By contrast, β-blockers, despite having served as the cornerstone of therapy for symptomatic oHCM for almost 6 decades, are supported by limited high-quality evidence. The most recent ESC (2023)8 and American College of Cardiology (ACC)/American Heart Association (AHA; 2024)9 guidelines continue to recommend non-vasodilating β-blockers as first-line therapy, despite the absence of robust comparative trials.
The MAPLE-HCM trial was conducted to evaluate the efficacy and safety of aficamten as monotherapy in comparison with metoprolol in patients with symptomatic oHCM.10,11
MAPLE-HCM: Overview and Key Findings
Study Design
MAPLE-HCM was an international, double-blind, double-dummy Phase III trial (Figure 1). Participants, aged 18–85 years with newly diagnosed or chronic oHCM, were randomly assigned to receive aficamten plus placebo or metoprolol plus placebo.10,11 Doses of metoprolol were uptitrated in 50 mg increments from 50 to 200 mg, while aficamten doses were titrated in 5 mg increments from 5 to 20 mg, guided by echocardiographic and clinical parameters.10,11 Patients on pre-existing therapy underwent a 2-week washout prior to screening.10,11
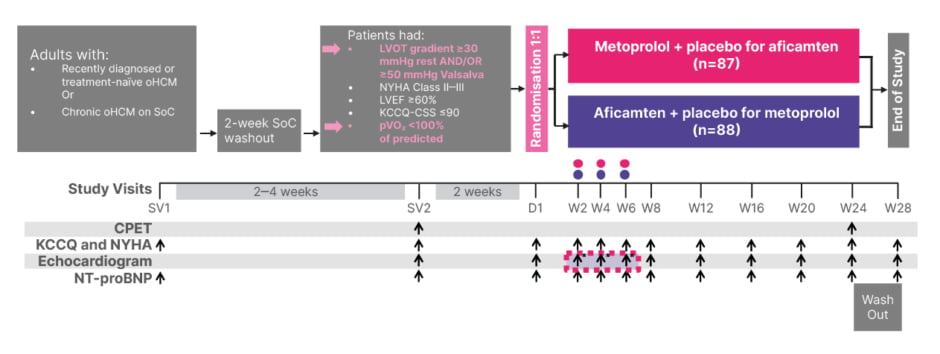
Figure 1: MAPLE-HCM study design.
*Metoprolol doses were uptitrated in 50 mg increments from 50–200 mg. Aficamten doses were uptitrated in 5 mg increments from 5–20 mg. Dose adjustment was based on site echo and vital signs.
Adapted from Garcia-Pavia P et al.10
CPET: cardiopulmonary exercise testing; D: day; KCCQ: Kansas City Cardiomyopathy Questionnaire; KCCQ-CSS: Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; oHCM: obstructive hypertrophic cardiomyopathy; pVO₂: peak oxygen uptake; SoC: standard of care; SV: screening visit; W: week.
The primary endpoint was the change from baseline to Week 24 in peak O2 uptake, as assessed during exercise testing. Secondary endpoints included an improvement from baseline to Week 24 of at least one New York Heart Association (NYHA) functional class, and a change from baseline to Week 24 in the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), NT-proBNP level, LVOT gradient after the Valsalva manoeuvre, and left ventricular mass index.10,11 Safety endpoints included a left ventricular ejection fraction (LVEF) <50%.11
Baseline Characteristics
In total, 175 patients were randomised.11 Overall, baseline characteristics reflected a milder phenotype compared with prior cardiac myosin inhibitor studies, including SEQUOIA-HCM.4 Hypertension was more prevalent in the aficamten group compared with metoprolol (61.4% versus 37.9% of patients, respectively).10
The majority of participants tolerated higher doses of the study medications, with 63% of patients randomised to metoprolol achieving doses of 150–200 mg, while 76% of those assigned to aficamten were maintained on doses of 15–20 mg.11 Haemodynamic effects reflected the expected pharmacology of the treatments, with a decrease in heart rate and systolic blood pressure of approximately 6 bpm and approximately 6 mmHg, respectively, by Week 24 among patients receiving metoprolol. No significant change in heart rate and a modest increase in systolic blood pressure (approximately 5 mmHg) was observed with aficamten.10
Primary Endpoint
Over the 24-week treatment period, patients treated with metoprolol experienced a reduction in peak O2 uptake of 1.2 (95% CI: 0.8–1.7) mL/kg/min, compared with a mean increase of 1.1 (95% CI: 0.5–1.7) mL/kg/min among patients treated with aficamten.10,11 The least squares mean (LSM) difference between groups was 2.3 (95% CI: 1.5–3.1) mL/kg/min, which exceeds the clinically relevant threshold of 1.0 mL/kg/min commonly applied in practice.10,11 This benefit was consistent across all pre-specified subgroups.10,11
Secondary Endpoints
Patients receiving aficamten showed greater improvements in all secondary endpoints (p<0.01 for all) except for left ventricular mass index.11 At Week 24, a total of 51% of patients in the aficamten arm demonstrated an improvement by at least one NYHA class compared with 26% in the metoprolol group (p<0.001).10,11 Moreover, 40% of patients in the aficamten group were asymptomatic (NYHA Class I) by study end, compared with only 9% in the metoprolol arm.10 Quality of life, as assessed by KCCQ-CSS, improved in both treatment groups, but the magnitude of benefit (LSM difference) was seven points greater with aficamten by Week 24 versus metoprolol (p=0.002).10
Haemodynamic differences between the two groups were particularly striking. No improvement in resting or Valsalva LVOT gradients was observed with metoprolol, despite a significant reduction in heart rate and blood pressure.10 In contrast, aficamten produced a rapid and profound reduction in LVOT gradients, with mean values falling below the range generally associated with relief of obstruction (clinically relevant threshold) by the study end (Figure 2).10,11 Favourable changes in biomarkers and structural indices were also observed. At Week 24, aficamten significantly decreased the NT-proBNP level, while this parameter remained elevated in the metoprolol group (81% reduction at Week 24 versus metoprolol; p<0.0001).10 Similar findings were reported for left atrial volume index, with an LSM difference of 7 mL/m2 in favour of aficamten versus metoprolol (p<0.0001) at Week 24.
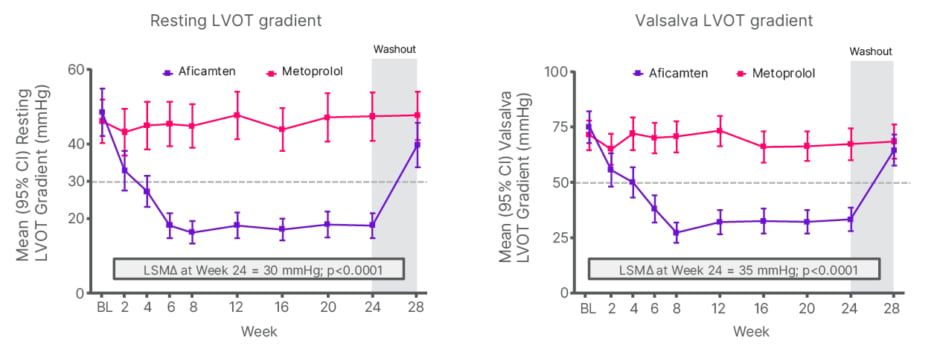
Figure 2: Secondary endpoints: change in left ventricular outflow tract obstruction at Week 24.
The grey dashed lines indicate the thresholds for resting (30 mmHg, left panel) and post-Valsalva (50 mmHg, right panel) LVOT gradient for oHCM.
Adapted from Garcia-Pavia P et al.10
BL: baseline; LSM: least squares mean; LVOT: left ventricular outflow tract; oHCM: obstructive hypertrophic cardiomyopathy.
Safety
Treatment with aficamten was well tolerated. Serious adverse events occurred in seven patients (8%) on aficamten and six patients (7%) receiving metoprolol. Three patients discontinued metoprolol due to adverse events, compared with one patient in the aficamten group. Dose reduction was required in 26 patients on metoprolol and in only four patients on aficamten.10,11 By Week 24, 10 patients were unable to tolerate metoprolol, and one patient was not able to tolerate aficamten.
Consistent with its mechanism of action, aficamten was associated with a modest reduction in LVEF (LSM between-group difference of −4.2 percentage points [95% CI: −5.3 to -3.1] at Week 24).11 However, values remained within the normal range for almost all participants; only one aficamten-treated patient had an ejection fraction <50%, and this individual remained asymptomatic without signs of heart failure.10,11
In summary, aficamten demonstrated superiority over metoprolol for the primary and multiple secondary endpoints, with clinically meaningful improvements in exercise capacity, symptoms, haemodynamics, and biomarkers, while maintaining an acceptable safety profile.10,11
Effect of Aficamten Compared with Metoprolol on Cardiac Structure and Function in Symptomatic Obstructive Hypertrophic Cardiomyopathy
A separate pre-specified analysis, presented by Hegde, further evaluated cardiac structural and functional parameters among patients in the MAPLE-HCM trial. As described by Garcia-Pavia, resting and Valsalva LVOT gradients declined significantly with aficamten, while remaining unchanged with metoprolol. LVEF decreased modestly in the aficamten group (approximately 5%) but remained well above the normal threshold (≥50%), reflecting a reduction in hypercontractility without the development of systolic dysfunction.10-12 In addition, patients treated with aficamten demonstrated a significant advantage in global circumferential strain and longitudinal strain, with between-group differences of −2.5% (95% CI: −3.7to -1.3) and −1.8% (95% CI: −1.8 to -0.5), respectively, while remaining within the normal range (Figure 3).12
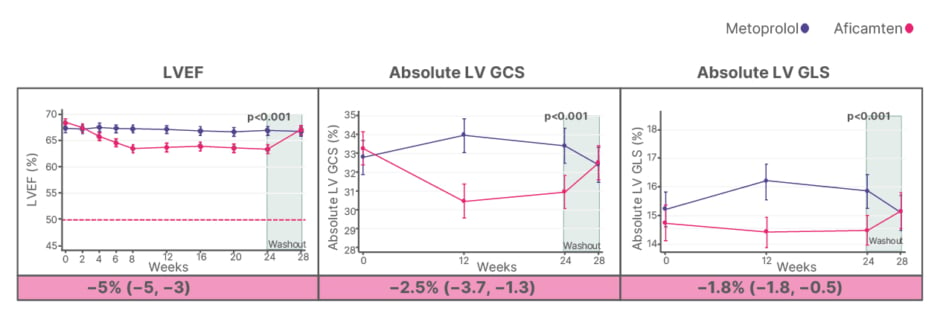
Figure 3: Systolic function and strain.
Treatment-corrected difference (95% CI) is adjusted for baseline echo measure, treatment, exercise mode (bicycle vs treadmill), and stratification by time of diagnosis (recent [Group 1] vs chronic [Group 2]) with corresponding p values at 24 weeks.
Horizontal dashed lines represent thresholds for gradients and normal values for LVEF.
Adapted from Hegde SM et al.12
GCS: global circumferential strain; GLS: global longitudinal strain; LV: left ventricular; LVEF: left ventricular ejection fraction; vs: versus.
Regarding structural remodelling, maximal wall thickness and inferolateral wall thickness declined modestly in the aficamten group compared with metoprolol (−1.0 mm [95% CI: −1.8 to -0.20] and −0.8 mm [95% CI: −1.5 to-0.0], respectively). In addition, the left ventricular end systolic volume index increased by 1.6 mL/m2 (95% CI: 0.7–2.5) in the aficamten group compared with metoprolol.12
Improved diastolic function was also observed following treatment with aficamten compared with metoprolol, as demonstrated by statistically significant improvements in left atrial volume index, as well as ratios of early mitral inflow velocity and septal and lateral mitral annular early diastolic velocity.12
Systolic anterior motion (SAM) of the mitral valve, a hallmark of oHCM, was present in most participants at baseline (83% metoprolol and 84% aficamten patients). After 24 weeks, aficamten significantly reduced the prevalence and severity of SAM compared with metoprolol (odds ratio [OR] versus metoprolol for SAM: 0.20 [95% CI: 0.08–0.52]; OR for SAM plus septal contact: 0.09 [95% CI: 0.04–0.24] at Week 24). Parallel improvements were observed in mitral regurgitation severity, whereby the proportion of patients in the aficamten group with moderate or severe regurgitation reduced from 39% at baseline to 25% at Week 24 (OR versus metoprolol: 2.49 [95% CI: 1.38–4.51] at Week 24).12
Overall, these findings demonstrated that, as well as improving exercise capacity and symptoms, aficamten achieved significant improvement in multiple measures of cardiac structure and function compared with metoprolol. The benefits of aficamten were evident as early as Week 2 after treatment initiation. Although there was a modest decline in measures of left ventricular systolic function with aficamten, all values remained within the normal range, reflecting reduced hypercontractility. Surprisingly, LVOT gradients were not effectively lowered with metoprolol despite physiologic evidence of adequate β-blockade.
Conclusion
The MAPLE-HCM trial provides the first head-to-head comparison of aficamten and metoprolol in symptomatic oHCM. Across both efficacy and structural endpoints, aficamten consistently demonstrated superiority, with significant and clinically meaningful improvements in exercise capacity, symptom burden, haemodynamic parameters, biomarkers, and cardiac structure.10-12 The safety profile was favourable, with only modest reductions in LVEF observed, which remained within normal limits.
Despite the historical reliance on β-blockers as first-line therapy for oHCM, the results of MAPLE-HCM indicate that aficamten may represent a more effective alternative, directly targeting the underlying pathophysiology of the disease. These data extend prior evidence and support the use of aficamten as a monotherapy or potential first-line therapeutic option in patients with oHCM, with potential implications for future treatment guidelines.13







