INTRODUCTION
Atopic dermatitis (AD) is a common and chronic skin condition that can be significantly impacted by the physiological and immunological changes of pregnancy. Hormonal changes in pregnancy induce an immunological shift to a Th2-dominant state, which may exacerbate pre-existing AD or manifest as de novo atopic eruption of pregnancy.1,2 Pregnant patients with AD face unique challenges as their condition may flare or evolve, particularly in the first two trimesters, and treatment decisions must balance maternal benefit with fetal safety. In the postpartum period, patients who are lactating encounter additional obstacles in therapeutic decision-making due to the potential excretion of drugs in breast milk that may ultimately pose risks to the infant. Given the rapid advancement of therapeutic options for AD, this feature discusses the safety considerations of dermatologic drugs during pregnancy and lactation, and provides recommendations for clinicians treating patients of childbearing age from pre-conception through postpartum.
TOPICAL MEDICATIONS
This section reviews the safety of topical medications for AD during pregnancy and lactation, with the authors’ recommendations summarised in Table 1.
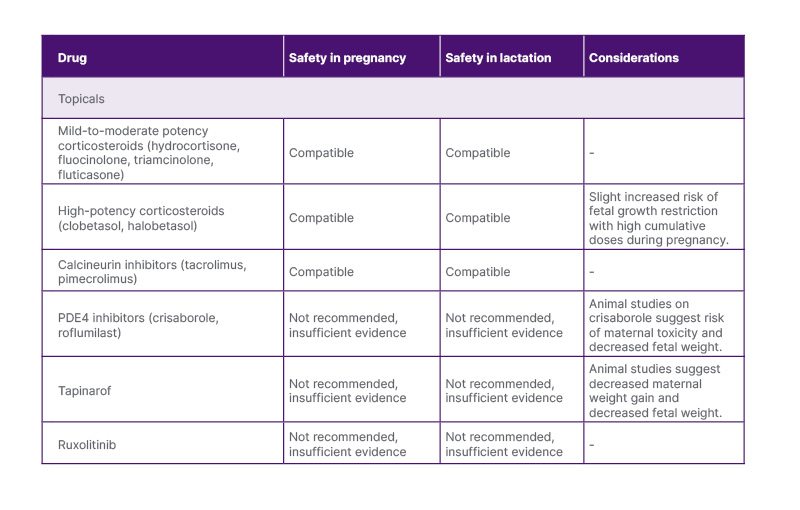
Table 1: Summary of topical therapies for atopic dermatitis during pregnancy.
PDE4: phosphodiesterase 4
Topical corticosteroids (TCS) are a mainstay treatment for AD exacerbation and proactive maintenance.3 The local side-effect profile of TCS, such as atrophy, striae, hypertrichosis, and telangiectasias, applies to both pregnant and non-pregnant patients.2 The risk of these side-effects increases with high potency, excessive use, and application to thin skin.3 Thus, it is recommended that patients use the lowest effective potency for the shortest possible duration. In pregnant and breastfeeding patients, in areas of rapidly expanding skin, such as the inner thighs, gravid abdomen, or breasts, TCS predispose to striae formation, so counselling regarding judicious use is helpful to prevent the development of striae. In terms of fetal safety, intermittent use of mild-to-moderate-potency TCS, including hydrocortisone, fluocinolone, and triamcinolone, during pregnancy has not been associated with fetal adverse events (AE).4 There is some evidence that very high cumulative doses (>300 g) of potent or ultrapotent corticosteroids, such as clobetasol or halobetasol, may be associated with fetal growth restriction (FGR), though recent large studies suggest low risk.4-6 During lactation, excretion of TCS in breast milk is minimal, reflecting the low maternal systemic absorption associated with topical use.7 There was a case of iatrogenic hypertension in an infant exposed to high-potency TCS applied directly to the nipple.7 Thus, high-potency TCS should be used with caution on the nipple and areola, where the infant may directly ingest the drug.7,8 Taken together, TCS are a safe first-line and adjunctive therapy for AD in pregnancy and lactation when used appropriately.1,2,4,7
Topical calcineurin inhibitors (TCI), such as tacrolimus and pimecrolimus, serve as steroid-sparing treatment options for AD. While there are limited data on TCI use during pregnancy or lactation, systemic tacrolimus use during pregnancy has not shown increased risk of congenital malformations. However, it has also been associated with prematurity, FGR, neonatal hyperkalaemia, and renal toxicity.2,4,9 Current evidence indicates that TCIs have poor systemic absorption, and the risk of fetal AEs is low.1,4 Pimecrolimus can be excreted in breast milk at high maternal serum concentrations, but transfer to the infant following topical application is expected to be negligible.9 Pimecrolimus cream may be preferred for nipple application during lactation due to the mineral paraffins found in tacrolimus ointment.10 TCIs are considered safe during pregnancy and lactation when used intermittently on a limited body surface area.2,4,7,11
Phosphodiesterase 4 (PDE4) inhibitors, including crisaborole and roflumilast, are another class of steroid-sparing anti-inflammatory agents used to treat AD. In animal studies, crisaborole was associated with maternal toxicity, decreased fetal viability, and low birth weight.4,12 There is no evidence to support the safety of topical PDE4 inhibitors in human pregnancy or lactation, and they are not recommended until more data become available.
Tapinarof is a novel aryl hydrocarbon modulating agent approved in the USA for the treatment of AD in children.13 Preclinical studies in rats showed decreased maternal weight gain, fetal viability, and birth weight with high doses.4,14 There is no evidence of tapinarof’s safety in human pregnancy or lactation, and it is not recommended until sufficient data emerge.
Topical ruxolitinib is a selective JAK1 and JAK2 inhibitor approved in the USA to treat AD.15 Preclinical animal studies did not demonstrate a risk of fetal malformations, though low fetal weight occurred with oral doses 22-times higher than the maximum topical human dose.16 No evidence exists regarding topical ruxolitinib safety in pregnancy or lactation, and the manufacturer recommends discontinuing 2–6 weeks before conception.4 Until further evidence emerges, it is not recommended during pregnancy or lactation.
LIGHT THERAPY
Narrowband UVB (NB-UVB) phototherapy is an effective treatment option for AD.17 NB-UVB poses no direct risk of teratogenicity; however, it can decrease serum folate levels in a dose-dependent manner in as few as seven sessions.18,19 As maternal folic acid deficiency is highly associated with neural tube defects, pregnant patients receiving NB-UVB are considered high-risk for deficiency and should receive folic acid supplementation between 1–5 mg/day.17 Psoralen plus UVA (PUVA) is occasionally used for severe, refractory AD. PUVA is contraindicated in pregnancy due to the mutagenic and teratogenic properties of oral psoralen.20 During lactation, no AEs have been reported with NB-UVB phototherapy.21 There are limited data on the safety of PUVA in lactation, but it is recommended for mothers to avoid breastfeeding for at least 24 hours after oral psoralen due to drug excretion in breast milk and risk of photosensitivity in the breastfed infant.22 NB-UVB is a safe therapeutic option during pregnancy and lactation, particularly when TCS alone are ineffective.1
SYSTEMIC MEDICATIONS
This section reviews the safety of systemic therapies for AD, including immunomodulators, JAK inhibitors, biologics, and antihistamines, during pregnancy and lactation, with the authors’ recommendations summarised in Table 2.
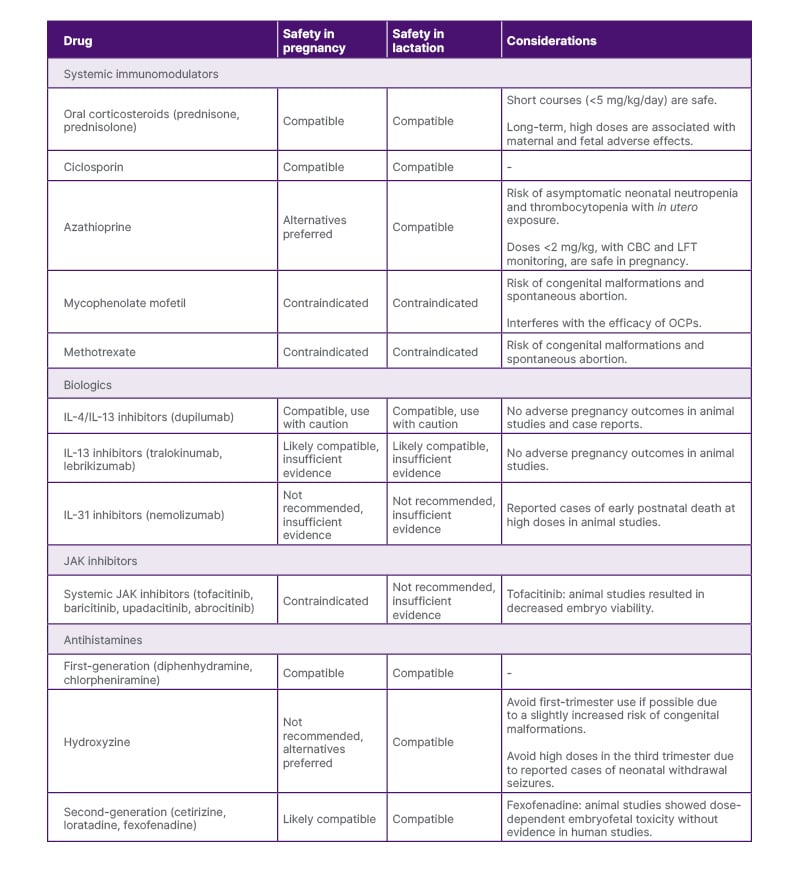
Table 2: Summary of systemic therapies for atopic dermatitis during pregnancy.
CBC: complete blood count; LFT: liver function test; OCP: oral contraceptives.
Systemic Immunomodulators
Oral corticosteroids, such as prednisone and prednisolone, can be useful for acute AD exacerbation. Prednisone and prednisolone at low doses (10–15 mg/day) during pregnancy confer minimal risk to the fetus, as they are converted to relatively inactive forms by 11β-hydroxysteroid dehydrogenase in the placenta.23-25 However, long-term treatment and higher doses increase the risk of fetal AEs (FGR, prematurity, and oral clefts with first-trimester exposure) and maternal AEs (premature rupture of membranes, gestational diabetes, hypertension, and pre-eclampsia with third-trimester exposure).2,4,25 The lowest effective dose for the shortest possible duration should be used in pregnancy to avoid these AEs, with a maximum dose of 0.5 mg/kg/day.1,2 Prednisone is excreted in breast milk, with peak levels achieved 1–2 hours after ingestion.11,26 With high doses of prednisone, lactating mothers should wait 4 hours before breastfeeding or pumping when maternal serum levels decrease substantially.7 Overall, oral corticosteroids are considered safe during pregnancy and lactation with appropriate use.
Ciclosporin, an oral calcineurin inhibitor, can be used off-label in the USA for treatment-resistant AD. In animal studies, high doses of ciclosporin (>10 mg/kg/day) in utero were associated with reduced nephron mass and renal insufficiency in offspring, but these findings have not been demonstrated in human studies.27-29 Data regarding ciclosporin use during pregnancy are primarily derived from organ transplant recipients and have been associated with increased risk of prematurity, FGR, Caesarean delivery, hypertension, and pre-eclampsia.4,24 However, it is difficult to discern whether these effects are due to ciclosporin exposure, underlying maternal comorbidities, or concomitant medications.6,27 Ciclosporin excretion in breast milk is highly variable, but blood levels in breastfed infants are typically undetectable.30 Case reports suggest no evidence of adverse effects on renal function or development in breastfed infants exposed to ciclosporin.11,30 With this, low-dose ciclosporin is considered safe during pregnancy and lactation when topicals or phototherapy fail, or when rapid disease control of severe AD is needed.1,2,11 During lactation, serum drug levels should be monitored in the breastfed infant.7
Azathioprine is a purine anti-metabolite that can be used off-label in the USA to treat refractory AD. Historically, azathioprine has not been recommended during pregnancy due to some evidence of increased risk of asymptomatic neonatal neutropenia and thrombocytopenia following in utero exposure.4,6,31 Multiple controlled studies suggest that azathioprine is not teratogenic, but some reports note an increased risk of FGR and prematurity.24,32 Azathioprine is found in small amounts in breast milk, and current evidence suggests no AEs in breastfed infants.33 There is concern for the potential risk of immunosuppression and carcinogenesis in breastfed infants exposed to azathioprine, but long-term follow-up studies have not been performed.2,33,34 When necessary, azathioprine at low doses (<2 mg/kg) can be used during pregnancy; however, maternal toxicity should be monitored with complete blood counts and liver function tests to reduce the risk of fetal complications.4,34 Azathioprine is considered safe during lactation, and it is recommended to wait at least 4 hours after the last dose to reduce the amount transferred to the breastfed infant.2,7,33
Mycophenolate mofetil (MMF) inhibits purine synthesis and can be used off-label in the USA to treat AD. MMF is associated with an increased risk of spontaneous abortion and congenital malformations, such as heart defects and facial clefts, with in utero exposure.4,6,35 Patients of childbearing age should use two reliable methods of contraception while on MMF and be informed that the drug may reduce the efficacy of oral contraceptives.4,36 MMF should be discontinued for at least 6 weeks before conception, and patients should continue to use reliable contraceptive methods during this time.6 There are limited safety data on MMF use during lactation, and it is considered high risk for immunosuppression in the breastfed infant.9,37 MMF is contraindicated during pregnancy and lactation.2,4,11
Methotrexate, a dihydrofolate reductase inhibitor, can be effective for refractory AD. Methotrexate is associated with an increased risk of congenital malformations and spontaneous abortion.1,2,24 Patients of childbearing age should discontinue the drug 1–3 months before conception and start high-dose folic acid supplements during this time through the first trimester to reduce the risk of neural tube defects.4,6,38 Although small amounts of methotrexate are detected in breast milk, there is a risk of accumulation of the drug in tissues and myelosuppression in breastfed infants.9,11,39 Methotrexate is contraindicated during pregnancy and lactation.2,4,40
Biologics
The advent of biologic therapy has improved patient outcomes and revolutionised the treatment of AD.41 During pregnancy, expression of placental neonatal Fc receptors allows for selective transplacental passage of maternal IgG antibodies, including therapeutic monoclonal antibodies.42 The placenta does not fully develop until approximately 12 weeks of gestation; thus, fetal exposure to monoclonal antibodies in the first trimester is negligible. Transplacental passage of monoclonal antibodies increases exponentially during the second and third trimesters as neonatal Fc receptor expression increases, with peak fetal exposure nearing parturition.4 There are a few reports of fatal tuberculosis infections following Bacillus Calmette-Guérin vaccination given at 3 months of life in infants exposed in utero to TNF inhibitors, specifically infliximab, adalimumab, and one unspecified TNF inhibitor.43,44 Due to the risk of neonatal immunosuppression following in utero biologic exposure, it may be beneficial to discontinue biologic therapy after 20 weeks of gestation. Clinicians should engage in shared decision-making with patients regarding continuation of therapy beyond 20 weeks, weighing the risk of disease flare versus the risk of neonatal immunosuppression. If biologic therapy is continued to term, decisions regarding live vaccination in exposed infants should be made in conjunction with a paediatrician or immunologist.
Dupilumab, an IgG4 monoclonal antibody, inhibits IL-4 and IL-13 signalling in AD by binding to the IL-4 receptor α subunit. Animal studies on dupilumab showed no evidence of pregnancy complications, congenital malformations, or neonatal immune dysfunction with in utero exposure.4,6 There are no large-scale human studies; however, multiple case reports and case series have not identified maternal-fetal complications, with all cases resulting in live births at term except for one premature delivery.45-51 A recent systematic review found no increased risk of congenital malformations or spontaneous abortion compared to the general population.52 Dupilumab has a high molecular weight, suggesting low transplacental passage, at least during the first two trimesters.9 Treatment in the third trimester may lead to fetal exposure, though the significance of this exposure is unknown. Dupilumab is unlikely to be detected in breast milk due to its large molecular size.53 Taken together, these data are reassuring, and dupilumab can likely be used in pregnancy and lactation when maternal benefit outweighs potential risk.1,53
Tralokinumab and lebrikizumab are IgG4 monoclonal antibodies that bind to IL-13, inhibiting its signalling pathway in the pathogenesis of AD. There is a lack of human data or case reports on tralokinumab or lebrikizumab use in pregnancy or lactation. Weekly administration of IL-13 inhibitors in preclinical animal studies did not result in adverse pregnancy outcomes, congenital malformations, or neonatal immunodeficiency.54,55 Tralokinumab and lebrikizumab are both large protein molecules, and the amount excreted in breast milk is presumably very low.56,57 IL-13 inhibitors are likely safe in pregnancy and lactation; however, more evidence is needed to give definitive recommendations.
Nemolizumab is a novel IgG2 antibody that targets pruritus in AD through selective inhibition of IL-31 receptor α. There is no evidence establishing the safety of nemolizumab in pregnancy or lactation. Animal studies did not demonstrate maternal or embryofetal toxicity at doses up to 36-times the maximum human dose, though three cases resulted in postnatal death soon after birth at this dose.58 Nemolizumab is a large protein molecule, and it is likely excreted in breast milk in small amounts.59 Given the lack of data and the novelty of the drug, the safety of nemolizumab in pregnancy and lactation remains unclear.
Small Molecule Inhibitors
JAK inhibitors are a relatively new class of drugs that have been shown to rapidly improve AD symptoms.60 The low molecular weight of JAK inhibitors may allow for transplacental passage early in pregnancy, and animal studies showed teratogenicity and risk of spontaneous abortion.60 For this reason, clinical trials excluded pregnant patients and required strict adherence to contraceptive methods during treatment.
Abrocitinib, a selective JAK1 inhibitor, and upadacitinib, a selective JAK1/2 inhibitor, are approved in the USA to treat AD. Other oral JAK inhibitors, such as tofacitinib and baricitinib, are occasionally used off-label to treat AD in the USA. In AD clinical trials, inadvertent exposure to abrocitinib or upadacitinib during pregnancy resulted in both healthy live births and cases of spontaneous abortion.60,61 Similarly, clinical trials and post-marketing surveillance studies on baricitinib revealed cases of healthy newborns, prematurity, and spontaneous abortion.60 Animal studies on tofacitinib resulted in decreased embryo viability and impaired structural development.62 A recent systematic review found that tofacitinib exposure in pregnancy led to healthy newborns, spontaneous abortions, and a few cases of congenital malformations; however, congenital malformations were present in cases with concomitant systemic drug exposure.63 Patients of childbearing potential should use reliable contraception while on these medications and for at least 4 weeks after stopping abrocitinib, upadacitinib, or tofacitinib, and for at least 1 week after discontinuing baricitinib.61,64 No evidence exists on the safety of abrocitinib, upadacitinib, or baricitinib in lactation. Limited data on tofacitinib suggest low concentration in breast milk without reported AEs in breastfed infants.65 Until there are sufficient data to definitively conclude the safety of systemic JAK inhibitors, these drugs are contraindicated in pregnancy and not recommended during lactation.
Antihistamines
First-generation antihistamines (FGA), including over-the-counter options like diphenhydramine and chlorpheniramine, are considered first-line for AD-associated pruritus in pregnancy.4,6 While one study associated first-trimester diphenhydramine use with cleft palate, no other reports of teratogenicity with non-prescription FGAs exist.9,66,67 High doses are not recommended near delivery as they may cause infantile sedation.3 Hydroxyzine, a prescription FGA, crosses the placenta and has been associated with a slightly increased risk of congenital malformations (5.8%) with no specific pattern of anomaly when used in the first trimester, as well as neonatal withdrawal seizures following prolonged maternal use in the third trimester.4,9,68 FGAs are transferred in breast milk, and high doses may decrease milk supply and cause drowsiness and irritability in the breastfed infant.69-71 Overall, diphenhydramine and chlorpheniramine are safe in pregnancy, and hydroxyzine should be used with caution.4,6 FGAs are safe during lactation, but low doses at bedtime are preferred to reduce AEs in the infant.69
Second-generation antihistamines, including cetirizine and loratadine, are considered acceptable alternatives to FGAs after the first trimester based on animal and human studies showing no significant maternal-fetal toxicity.6,72 While there are minimal data on the safety of fexofenadine in human pregnancy, preclinical studies found a dose-dependent embryofetal toxicity with first-trimester exposure in rats.9 Loratadine and fexofenadine are found in low levels in breast milk and are non-sedating.73,74 Large doses of cetirizine may cause drowsiness in the breastfed infant.75 Due to more comprehensive safety data, FGAs are preferred in pregnancy.9 Second-generation antihistamines, particularly loratadine and fexofenadine, are safe in lactation.39,73-75
CONCLUSION
Effective management of AD during pregnancy and lactation requires careful consideration of maternal benefits and the wellbeing of the child. Evaluating the risk–benefit ratios of available dermatologic therapeutics is crucial in the shared decision-making for this patient population. Treatment of AD during pregnancy and lactation should be multidisciplinary, weighing the expert opinion of dermatologists, obstetricians, and paediatricians. Continued clinical trials, patient registries, and pharmacovigilance studies are essential to refine treatment strategies and ensure optimal outcomes for both mother and child.






