Abstract
Key Points
1. Deucravacitinib, a selective tyrosine kinase 2 inhibitor, shows promising efficacy in the treatment of refractory chilblain lupus, a rare subtype of cutaneous lupus erythematosus (CLE), offering a potential therapeutic option for patients unresponsive to conventional therapies.
2. The patient experienced significant clinical improvement within 4 weeks of initiating deucravacitinib monotherapy, with sustained remission over 2 years, demonstrating the drug’s potential for rapid symptom control and long-term disease management in CLE.
3. This report contributes to the growing body of literature supporting deucravacitinib’s efficacy across CLE subtypes and suggests that further studies are warranted to establish its role as a targeted therapy in autoimmune skin diseases beyond psoriasis.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition that affects multiple organ systems, with a strong predilection for women of childbearing age. This female predominance is thought to be influenced by hormonal factors, including the immunomodulatory effects of oestrogen, which may contribute to heightened autoimmune susceptibility. Cutaneous manifestations occur in up to 85% of patients with SLE.1 Conversely, cutaneous lupus erythematosus (CLE) is defined by disease limited to the skin without systemic involvement, and can occur as an isolated entity or as the initial manifestation of SLE. Chilblain lupus is a rare, chronic subtype of CLE characterised by painful, violaceous nodules with central ulceration, which are often triggered or exacerbated by cold exposure.2 Deucravacitinib is a selective tyrosine kinase 2 (TYK2) inhibitor approved for moderate-to-severe plaque psoriasis with ongoing clinical trials for SLE.3-5 Current data from both clinical trials and case reports in the literature suggest that deucravacitinib holds promise as an emerging therapeutic option in SLE and CLE. This article describes a case of lupus chilblains treated with deucravacitinib and reviews the promising role of deucravacitinib in CLE and SLE.
CASE DESCRIPTION
A 72-year-old woman with a past medical history of hypertension, asthma, coronary artery disease, prediabetes, and pernio of the toes presented to the dermatologist with painful, erythematous-to-violaceous acral lesions with tender fingertip ulcerations and onycholysis. The patient denied associated joint pain, fatigue, myalgia, or photosensitivity.
The patient underwent an extensive diagnostic work-up over the course of 6 years. Complete blood count revealed mild anaemia and baseline thrombocytosis. Autoimmune serologic studies revealed negative antinuclear, anti-double-stranded DNA, anti-ribosomal P, anti-topoisomerase I, thyroid peroxidase, anti-Smith, anti-ribonucleoprotein, anti-Sjögren’s-syndrome-related antigen A, anti-Sjögren’s syndrome B, and myositis antibody panels, as well as negative rheumatoid factor and normal complements (C3/C4). Serum protein electrophoresis and immunofixation were normal. A prior skin biopsy of the toes showed vacuolar interface dermatitis.
The patient had minimal relief with a range of topical therapies in the past, including clobetasol, mupirocin, nitroglycerin, betamethasone, and minoxidil. Systemic therapies such as nifedipine, doxycycline, and hydroxychloroquine were ineffective, and the patient was unable to tolerate pentoxifylline therapy due to adverse gastrointestinal effects.
Development of onychodystrophy prompted potassium hydroxide testing of the nails, which revealed fungal hyphae. The patient was prescribed oral fluconazole 100 mg daily for the first 7 days of each month, daily vinegar soaks, and topical tacrolimus on weekdays and clobetasol on weekends. The patient’s condition remained refractory, and the ulcerations on their hands interfered with activities of daily living.
At initial dermatologic consultation, bacterial cultures of the fingertips grew Pseudomonas aeruginosa and Serratia marcescens. A 10-day course of ciprofloxacin 500 mg twice daily provided modest improvement. The patient was started on a trial of deucravacitinib 6 mg daily. Within 4 weeks, the ulcerations on their fingertips fully re-epithelialised with marked improvement of onychodystrophy. At this point, the patient reported a 90% reduction in associated pain and swelling of the fingers. They remained on deucravacitinib for the subsequent 2 years without ulcer recurrence, pain, or adverse effects. The patient’s quality of life improved substantially, and no further complications were noted.
DISCUSSION
Lupus chilblains, also known as pernio, is a chronic inflammatory skin condition characterised by painful, erythematous, or violaceous lesions typically affecting acral sites including the toes, fingers, and ears.2 It is thought to be an abnormal vascular response to cold, leading to localised vasculitis. Lupus chilblains can occur sporadically or secondary to autoimmune conditions like SLE. Like other forms of connective tissue diseases, lupus chilblains is seen more frequently in women, likely due to the complex interplay between sex hormones, immune regulation, and vascular reactivity. Management is often challenging, and first-line management focuses on lifestyle modifications such as strict avoidance of cold exposure, photoprotection, and smoking cessation. Topical corticosteroids and systemic vasodilators such as nifedipine can provide symptomatic relief by reducing inflammation and improving peripheral circulation. Hydroxychloroquine is frequently used to treat chronic or autoimmune-related lupus chilblains, though outcomes are variable.6 Patients may see some improvement with these therapies, but relapse rates remain high.
Deucravacitinib, an oral, selective TYK2 inhibitor, is approved for the treatment of moderate-to-severe plaque psoriasis. TYK2 is involved in the regulation of IL-12, IL-23, and Type 1 interferons. Deucravacitinib is currently under investigation for the treatment of CLE and SLE. In the Phase II PAISLEY trial on adults with active SLE, deucravacitinib treatment resulted in higher response rates on the SLE Responder Index 4 (SRI-4) compared to placebo, with a favourable side-effect profile.3-5 In addition to this, in a recent systematic review, deucravacitinib demonstrated superior efficacy and safety with fewer side effects compared to anifrolumab in CLE.7
To date, nine cases of treatment-resistant CLE successfully treated with deucravacitinib have been documented in the literature Table 1, with most cases reported in female patients.8-15 Four cases describe the efficacy of deucravacitinib as adjunctive therapy to concomitant hydroxychloroquine or chloroquine, while five cases demonstrated significant clinical improvement with deucravacitinib as systemic monotherapy. In all cases, patients achieved rapid symptomatic relief and either complete clearance or minimal residual disease within a year of initiating deucravacitinib. The drug was well-tolerated across reports, with one case noting a mild inflammatory acneiform eruption that was managed with topical clindamycin.8 The literature indicates efficacy for multiple CLE subtypes, including discoid and tumid lupus erythematosus.8,9,12-14 Two cases, presented in Table 1 as Case 1 and Case 3, underscore the utility of deucravacitinib in patients with CLE with concurrent psoriatic disease, reinforcing its FDA-approved indication.8,15 The growing number of cases reporting successful use of deucravacitinib in treatment-refractory cutaneous lupus support its potential as an effective option for refractory cases.
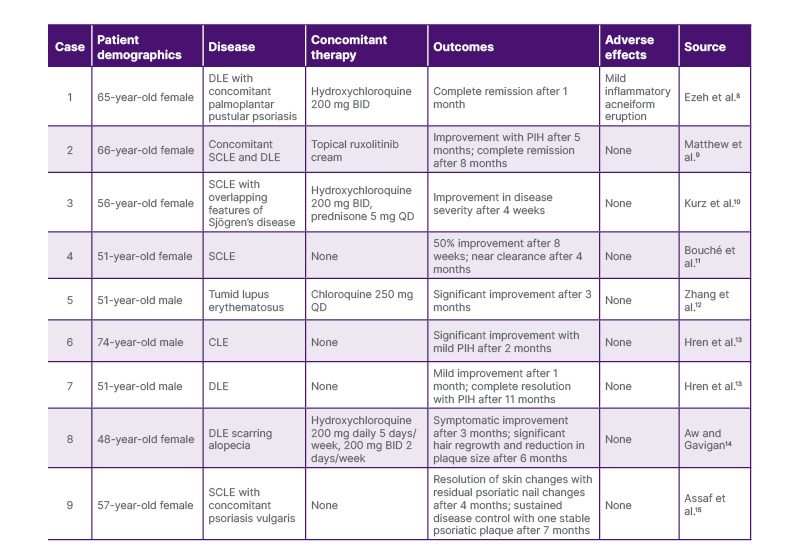
Table 1: Documented cases of cutaneous lupus erythematosus successfully treated with deucravacitinib.
BID: twice daily; CLE: cutaneous lupus erythematosus; DLE: discoid lupus erythematosus; PIH: post-inflammatory hyperpigmentation; QD: once daily; SCLE: subacute cutaneous lupus erythematosus.
To the authors’ knowledge, this article reports the first case of recalcitrant lupus chilblains successfully treated with deucravacitinib monotherapy. This case adds to the emerging literature on the promising use of deucravacitinib in CLE and highlights its potential as a targeted therapy for chilblain lupus, a rare and difficult-to-treat subtype of CLE. Further prospective studies are warranted to assess the long-term efficacy and safety of deucravacitinib in CLE.






