BACKGROUND AND AIMS
Anogenital lichen planus (ALP) is a chronic inflammatory condition associated with significant morbidity, persistent symptoms, architectural changes, and reduced quality of life.1-3 ALP can give rise to cutaneous squamous cell carcinoma (cSCC), and specifically in the setting of chronic inflammation, to Marjolin’s ulcer (MU), a rare, aggressive subset of cSCC associated with a poor and potentially life-threatening prognosis.4 The true incidence of ALP is likely underestimated due to the lack of routine genital examination by healthcare providers and the potentially asymptomatic nature of lesions.5 This systematic review aims to characterise the clinical characteristics of patients developing MU within ALP and its associated outcomes.
MATERIALS AND METHODS
Medline and Embase were searched from inception–November 2024, identifying English-language studies reporting MU or squamous dysplasia arising within ALP lesions in adult patients (≥18 years). Thirty-two studies (n=176 cases) were analysed, including cohort studies (n=19), case series (n=2), and case reports (n=11), and a descriptive analysis was performed.
RESULTS
The mean patient age was 63.9 years, with 77.27% being female (Table 1). ALP lesions were most commonly vulval (72.2%) in females and penile (22.2%) in males. Multiple clinical types of ALP aside from the classic presentation (papules and plaques) were observed, including erosive (54.43%; n=43/79) and hypertrophic (16.45%; n=13/79) subtypes. MU or cSCC precursor prevalence in ALP cohorts ranged from 0.9–4.2%. Forms of squamous dysplasia included MU (60.23%), differentiated-vulval or penile intraepithelial neoplasia (13.64%), undifferentiated-vulval or penile intraepithelial neoplasia (5.11%), or unspecified cSCC in situ (11.36%), with 9.66% of patients exhibiting multiple overlapping subtypes. Human papillomavirus (HPV) was rarely detected (8.41%), suggesting other mechanisms underlying MU development. The mean ALP duration prior to squamous dysplasia was 47.5 months; many patients reported symptoms of anogenital pruritus, pain, erythema, and dyspareunia occurring for years prior to diagnosis. Metastases were present in 50% of cases that reported this outcome. MU treatment primarily involved surgical excision (87.13%), with a few patients requiring radiation treatment (3.96%) or chemotherapy (1.98%). Recurrence with survival occurred in 19.74% of cases and mortality was reported in 21.05%. Of the patients who had a mortality outcome, all were female, with a mean age of 73.1 years and a diagnosis of cSCC rather than a precursor. The majority of cases had prior erosive ALP (56.25%; n=9/16) and none had a positive HPV status. Poor disease control, erosive morphology, and long-standing lesions were associated with higher malignancy risk. Cohort studies following patients with lichen planus (which only involved female patients) demonstrated new lesion rates of 0.9–4.2% for MU and 1.0–8.2% for vulval intraepithelial neoplasia during follow-up.
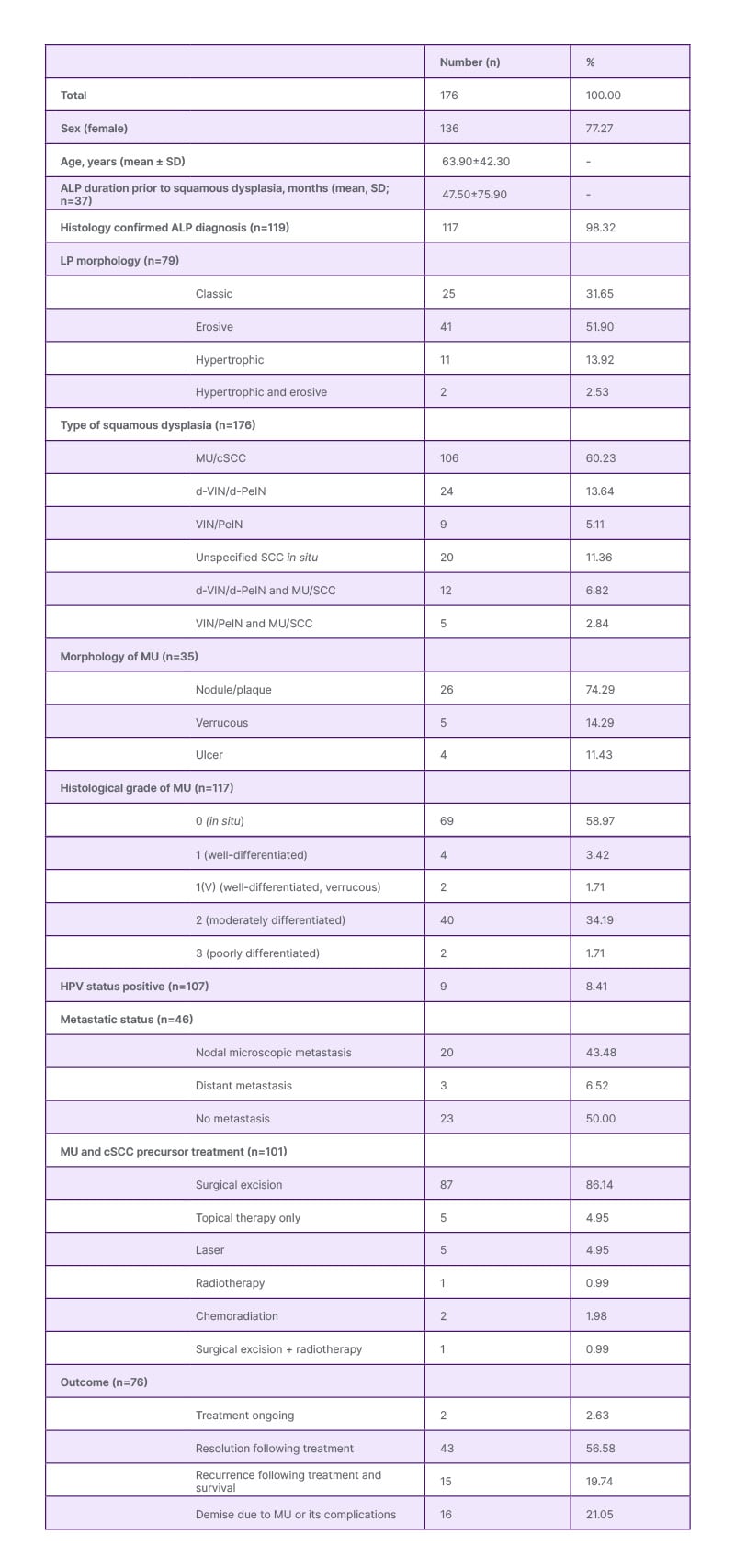
Table 1: Demographic and clinical characteristics of patients with anogenital lichen planus and cutaneous squamous cell carcinoma.
ALP: anogenital lichen planus; cSCC: cutaneous squamous cell carcinoma; d-PeIN: differentiated penile intraepithelial neoplasia; d-VIN: differentiated vulval intraepithelial neoplasia; HPV: human papillomavirus; LP: lichen planus; MU: Marjolin’s ulcer; PeIN: penile intraepithelial neoplasia; SCC: squamous cell carcinoma; VIN: vulval intraepithelial neoplasia.
CONCLUSION
This review highlights the malignant potential of ALP and underscores the importance of early detection and multidisciplinary care. Malignant transformation may be related to the immunocompromised cutaneous district seen in ALP.6-8 Routine anogenital examinations, timely biopsies of suspicious lesions, HPV vaccine administration, and aggressive management of ALP may improve outcomes.5,9 The term MU for ALP-associated cSCC is justified by high recurrence and mortality rates, and consistent use of this terminology may better reflect its aggressive nature. Further research is needed to elucidate the mechanisms underlying MU development and optimise treatment strategies for this rare but morbid complication.






