Meeting Summary
This article summarises the symposium on atopic dermatitis (AD) recorded at the European Academy of Dermatology and Venereology (EADV) Congress, which took place from the 17th–20th September 2025 in Paris, France. The aim was to bring a guideline perspective to the treatment of AD through presentations by leading experts in disease management. AD is driven by skin barrier dysfunction, leading to allergen penetration, chronic inflammation, and an ongoing cycle of immune activation with Th2 cytokines that perpetuate itching and further barrier damage. Treatment satisfaction remains low, so it is important to fully understand who is a ‘moderate’ patient and the treatment options that are available to improve symptoms and quality of life (QoL).
Bridging The Gap: Evolving Moderate Atopic Dermatitis Care
Introduction
AD is one of the most common inflammatory skin conditions,1-4 with global prevalence estimated to be 2.6% (adults: approximately 2.0%; children: approximately 4.0%), and rates vary geographically.4,5 AD is commonly classified as mild, moderate, or severe based on the extent of skin involvement, intensity of symptoms, degree of itch, course of flare-ups, and scoring systems such as the Eczema Area and Severity Index (EASI), Investigator’s Global Assessment (IGA), body surface area (BSA) affected, and Scoring Atopic Dermatitis (SCORAD). Moderate AD usually presents with more widespread lesions, persistent disease activity, and greater impact on QoL compared to mild forms.2 Key characteristics of moderate AD are widespread lesions, frequent flare-ups and exacerbations, significant itch, and sleep disturbance, with patients having impaired QoL and daily functioning.2 Recognising the key clinical characteristics of moderate AD is crucial for providing appropriate and comprehensive management of this patient population.
Topical agents for AD must penetrate the skin barrier, with optimal efficacy influenced by characteristics including molecular size and lipophilicity. Accordingly, newer small-molecule agents offer promise for more successful topical therapy.6 Globally, treatment options are shaped by the economic context, with limited access in low-resource settings, but the recent inclusion of emollients on the WHO essential medications list is a significant advance.7 AD poses a high burden on patients’ QoL and healthcare systems, particularly in moderate-to-severe cases, and management should be tailored to disease severity, QoL impact, and patient preference using stepped-care guidelines for topical and systemic therapies.
Guidelines for Atopic Dermatitis Management
The North American 2018 treatment algorithm for the management of AD provides information for acute and maintenance treatment.8 The Canadian guideline9 adds an emphasis on chronicity, while the European guideline10,11 expands on systemic therapy pathways.
Both the Canadian and Europeanguidelines recommend:
- Confirming AD diagnosis and assessing severity/extent (e.g., BSA, QoL, anatomical sites).
- Starting with optimised skin care and patient education.
- Using topical therapies adjusted to severity (low-potency for mild, higher for more severe cases).
- Monitoring response
- 4–6 weeks specific to Canadian guideline.
- Stepped-care approach discussed by European guideline.
- Escalating to alternative topicals, phototherapy, or systemic therapy if control is inadequate.
- Incorporating shared decision-making and regular review of adherence and goals.
Defining the Unmet Needs and Patient Experience of Moderate Atopic Dermatitis
Chih-Ho Hong
A well-established problem is skin barrier dysfunction, which allows allergens and pathogens to infiltrate the epidermis, creating an inflammatory response. Cytokines triggered in the pathogenesis of AD require an intracellular JAK inhibitor-signal transducer and activator of transcription proteins (JAK-STAT) pathway to mediate inflammation and transmit itch signals. It is well known that many relevant cytokines in AD are controlled through JAK1 and JAK2 signalling;12-18 for instance, the IL-13 pathway that drives AD, and IL-31 (relevant for itch), both require JAK1 and JAK2 for signalling. Many other essential cytokine pathways, such as IL-22 (relevant for epidermal hyperplasia), signal through JAK1; thymic stromal lymphopoietin (TSLP) signals through JAK1 and JAK2; IL-5, which drives eosinophils, signals through JAK2; and interferon-γ signals through JAK1 and JAK2.
There are high rates of uncontrolled AD reported, with most patients stating itch as the most burdensome symptom of AD, followed by dryness and red or inflamed skin.19 Analyses of treatment satisfaction in adults and adolescents using topical (adults: n=284; adolescents: n=114) and topical + systemic (adults: n=110; adolescents: n=30) agents revealed that up to 50% of physicians were less than satisfied with the current level of disease control.20 Adult and adolescent patients surveyed who were using topical (adults: n=152; adolescents: n=80) and topical + systemic (adults: n=65; adolescents: n=17) agents also reported that they were less than satisfied with their disease control (adults: up to 30.8%; adolescents: up to 35.3%).20
Identifying the Patient With Moderate Atopic Dermatitis
There is an inconsistency in the definition of who is a ‘moderate’ patient, and it is crucial to understand where the terms originate to avoid incorrect use. The approval of topical tacrolimus ointment some 20 years ago for moderate-to-severe AD relied on the Rajka and Langeland criteria for defining disease severity.21,22 Here, three domains, extent, disease course, and intensity of itch, are evaluated to produce a score where 6–7 is moderate and 8–9 is severe. However, it may be challenging to conceptualise what this looks like in the clinic.
Modern topicals such as pimecrolimus, crisaborole, and ruxolitinib cream use a more standardised global assessment, where a moderate score is 3.23-29 Additionally, for those in the patient population who are moderate-to-severe and receive systemic agents like biologics or a systemic JAK inhibitor, moderate is identified by having an EASI score of ≥16, ≥10% BSA, and an IGA score of 3 (moderate) or 4 (severe).26-28 Such inconsistency leads to conflicting and different definitions of who is termed ‘moderate’. It is essential to understand these differences when reviewing clinical trials, and for dermatologists to appreciate that it is not necessarily a uniform patient population being discussed.
A Spanish study, MODERMYS-ES, is a cross-sectional survey on the management of moderate AD post-topical corticosteroid (TCS)/topical calcineurin inhibitor (TCI) treatment (N=300). A total of 100 specialists (dermatologists and allergists) treating primarily AD completed questionnaires on treatment shares, symptoms, and disease severity, looking at the percentage of BSA affected, IGA and EASI scores, disease onset, and comorbidities (de Frutos et al., unpublished data). Most patients were between the ages of 18–50 years (18–30 years: n=135 [45%]; 31–50 years: n=140 [47%]). Over 50% of patients were male (n=168; 56%) and most patients had had the disease since they were infants or children. A high percentage of patients had Type 2 comorbidities, including allergic rhinitis (n=145; 48%), asthma (n=139; 46%), and food allergy (n=64 [21%]; de Frutos et al., unpublished data). The face and hands were most affected (face: n=219 [73%]; hands: n=213 [71%]), almost 80% (n=169) of patients had less than 20% BSA affected, and 44% (n=133) had uncontrolled symptoms (de Frutos et al., unpublished data).
However, where BSA is less than 20%, it is difficult to have an EASI score >16. A typical patient with a similar BSA would have an EASI score of around half. Such patients would not be biologically eligible for reimbursement (in Canada). In addition to sleep disturbance, reduced productivity, and difficulties in daily activities, the visible nature of the condition can result in social stigma and emotional distress, further impacting the patient’s QoL.
Hong considered how an adult patient with chronic AD, an IGA score of 3, 11% affected BSA, and an EASI score of 10 might be currently treated, and how they might be treated post-TCS and -TCI when they are not eligible for biologic or oral JAK inhibitors.
Referring to the MODERMYS-ES study, he showed that cyclosporine was the most common treatment for patients with moderate AD after TCS and TCI, followed by systemic glucocorticosteroids (de Frutos et al., unpublished data). Of a cohort of 300 patients, the largest number were on combined therapies, which included the use of topicals with biologics (25%), conventional systemics (19%), and JAK inhibitors (9%; de Frutos et al., unpublished data).
For patients who are amenable to topical therapy, severity should be established based on clinical signs (erythema, oedema, post-inflammatory hyperpigmentation, excoriation, and lichenification), the extent of disease (%BSA), and patient-reported symptoms, notably itch.9 Initial therapy depends on whether the patient has more severe disease or mild-to-moderate disease (Figure 1). Once patients reach an optimum stage of disease control, treatment should be continued. If disease control is not achieved, patient adherence should be assessed, and the dermatologist should rule out other diagnoses. The current treatment can be adjusted or switched to a topical therapy, phototherapy, or systemic therapy.9
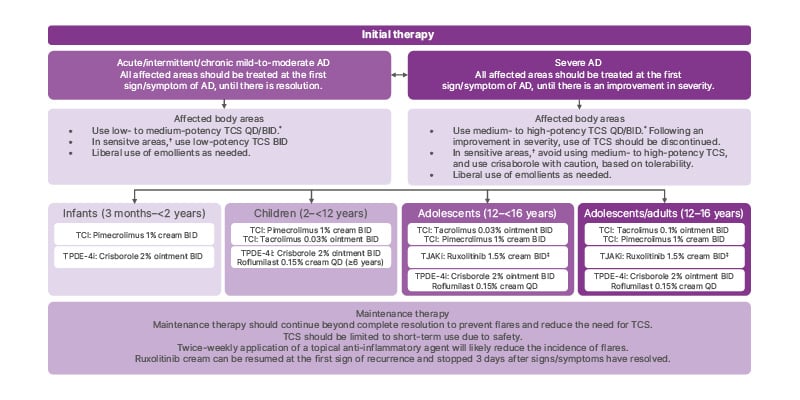
Figure 1: Algorithm for the topical therapy of atopic dermatitis in children, adolescents, and adults.
*Cochrane review has shown that QD application of TCS is as effective as BID application of TCS.
†Sensitive areas include the face (eyelids and perioral region), neck, axillary/inguinal region, and genital region.
‡Ruxolitinib (1.5% cream) can be resumed at the first sign of recurrence and stopped 3 days after signs/symptoms have resolved.9
AD: atopic dermatitis; BID: twice daily; QD: once daily; TCI: topical calcineurin inhibitor; TCS: topical corticosteroid; TJAKi: topical JAK inhibitor; TPDE-4i: topical phosphodiesterase-4 inhibitor.
Navigating Treatment Gaps and Key Challenges for Patients with Moderate Atopic Dermatitis
Andreas Wollenberg
When treating a skin disease like AD with a topical agent, the degree of penetration through the epidermal barrier is dependent on the physical characteristics of a substance. For therapeutics, the molecular weight of the agent (optimum size ≤500 Daltons) and other factors, including the extent of lipophilicity, are highly relevant.6 For example, tacrolimus (although larger, at approximately 800 Daltons) can be used topically because the epidermal barrier is disturbed in AD when compared with normal human skin or psoriasis.30,31 Newer agents, such as ruxolitinib cream or crisaborole (both approximately 300 Daltons), are much smaller, and it is reasonable to consider these molecules as an alternative strategy when considering treatment options.
The global treatment landscape for AD is influenced by several factors, the most important being economic.32,33 In Europe, the total cost of moderate-to-severe AD in adults is estimated to be approximately 30 billion EUR per year, with emotional impact and sleep deprivation adding further weight to indirect costs.34 In 2024, a position statement reported that the AD guidelines were not adapted for low-resource settings, highlighting that the number of people to be served across different countries is highly variable.35
Treatment options in high resource settings are diverse, from emollients (humectant, occludent, non-medicated options), TCS, and TCI, 8,32,36-41 to topical preparations and phototherapy (more accepted in Europe than the USA).32,42 In low-resource settings, treatment options are emollients (humectant and occludent), TCSs, and some TCIs if affordable, topical detergents, or wet wraps. In a big step forward for global patient care, the International Society for Atopic Dermatitis (ISAD) has recently been successful in adding emollients to the WHO list of essential medications for treating AD.7 Dermatologists must, therefore, keep in mind that the problems to be solved are different for each patient and are full of subjective burden regarding specific treatment options.
The European guideline (Figure 2) also contains useful definitions and information about treatment goals for patients with AD, including short-term and long-term therapies, reactive versus proactive treatment, and the clinical definition of flares.10
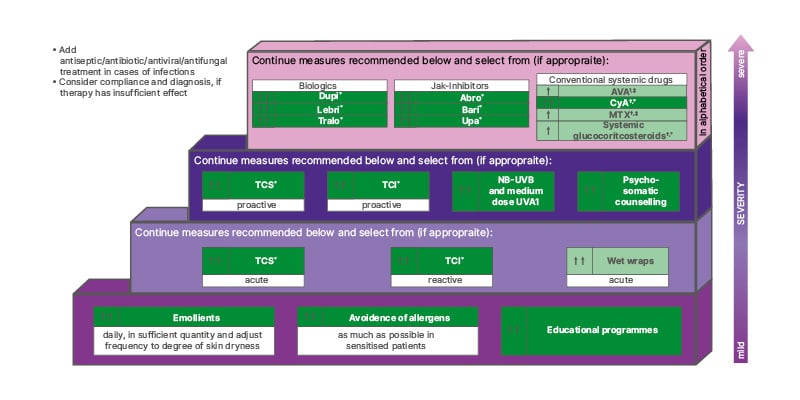
Figure 2: EuroGuiDerm guideline on atopic eczema stepped-care plan for adults with atopic eczema.
*Refer to guideline text for licensed indication, ?restrictions, and ‡off-label treatment.
Dark green boxes indicate strong recommendation for the use of an intervention. Light green boxes indicate weak recommendation for the use of an intervention. For definitions of disease severity (acute, reactive, and proactive) see section VII and the ‘Introduction to Systemic Treatment’ section of the EuroGuiDerm Atopic Eczema Guideline.11
Abro: abrocitinib; AZA: azathioprine; bari: baricitinib; CyA: ciclosporin; dupi: dupilumab; lebri: lebrikizumab; MTX: methotrexate; TCI: topical calcineurin inhibitors; TCS: topical corticosteroids; tralo: tralokinumab; upa: upadicitinib; NB-UVB: narrow band UV B.
Ultimately, patient adherence is influenced by the choice of treatment and the interaction between physician and patient.43 For patients, the itch is the most burdensome part of their disease, and most care about achieving symptom relief, especially itch, before complete clearance of their physical lesions.
Reshaping the Treatment Paradigm of Moderate Atopic Dermatitis: Clinical Trial Results for Ruxolitinib
José-Manuel Carrascosa
There are multiple cytokines involved in AD that require the JAK-STAT pathway to mediate inflammation and transmit itch signals. This presentation reviewed some clinical trial results for ruxolitinib, a selective JAK1 and JAK2 inhibitor.
Topical Ruxolitinib Evaluation in Atopic Dermatitis: TRuE-AD144 and TRuE-AD245
Eligible patients were at least 12 years of age with a minimum of 3 years’ history of AD, diagnosed as mild-to-moderate, and had an IGA score of 2 or 3 with BSA 3–20% (excluding scalp). Patients were randomised 2:2:1 to receive two doses of ruxolitinib, 1.5% twice daily and 0.75% twice daily for 8 weeks, with no rescue treatment permitted during this period. At 8 weeks, patients with an IGA 0–4 score could enter the long-term safety period. Patients initially randomised to ruxolitinib remained on their original regimen, and those on vehicle were randomised 1:1 to either 1.5% or 0.75% ruxolitinib twice daily. Treat-as-needed through Week 52 was performed, with treatment stopped 3 days after lesion clearance and restarting with lesion recurrence. Again, no rescue treatment was permitted, and there were site visits every 4 weeks.
The distribution of clinical characteristics was similar across treatment groups. The total population had a mean BSA of 9.8% (±5.4) and a baseline EASI of 8.0% (±4.8), and 75.0% of patients had an IGA score of 3. A total of 63.9% of patients had an Itch Numeric Rating Scale (NRS) score ≥4, and many patients (38.8%) had facial involvement. Mean flares in the past 12 months were 5.9, indicating this to be a patient population that remains in need of constant therapy. Notably, 90% of patients had received prior therapies for AD, which included different potencies of TCS (low: 49.6%; medium: 42.4%; high: 32.7%), TCIs (21.5%), and systemic corticosteroids (17.5%). Most treatment benefit was observed within the first 4 weeks. Significantly more patients who applied ruxolitinib cream achieved the primary endpoint of IGA-treatment success (IGA-TS), defined as an IGA score of 0 (clear) or 1 (almost clear) with at least a 2-point improvement from baseline. At 8 weeks, the greatest improvements were achieved by patients administered with 1.5% and 0.75% ruxolitinib (54.0% and 51.0%, respectively; p<0.0001) when compared with the vehicle group (7.6–15.1%).
Sub-analyses were performed to identify a moderate AD profile population defined by BSA ≥10% or EASI ≥16 at baseline. At Week 8, more patients in the moderate AD group achieved IGA-TS with ruxolitinib cream compared with vehicle. Improvements in EASI-75 (defined as achieving ≥75% improvement in EASI score) and Itch NRS were consistent across both TRuE-AD1 and TRuE-AD2 studies, and significantly more patients who applied ruxolitinib cream achieved EASI-75, a clinically meaningful improvement in itch compared with vehicle (p<0.05). In patients with moderate AD (BSA ≥10% and EASI ≥16 at baseline), ruxolitinib cream appeared to be highly efficacious.
Results from the long-term safety period (IGA) showed that the proportion of patients who achieved clear or almost clear skin was maintained throughout, with ruxolitinib used when needed. After 52 weeks, between 74–78% of patients achieved IGA 0–1 when the topical therapy was used, and the data suggest that ruxolitinib cream may delay or prevent the need for systemic treatment in a subset of patients with moderate AD profile (IGA score of 3, BSA ≥10%, and EASI ≥16 at baseline; Figure 3).
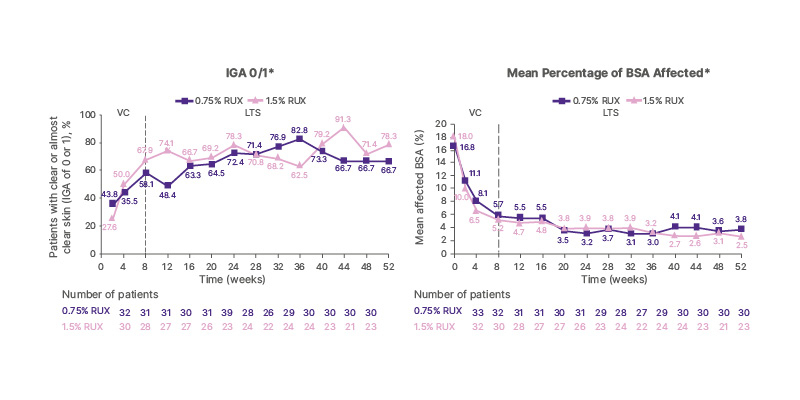
Figure 3: TRuE-AD 1 and 2 study sub-analyses: proportion of patients with moderate atopic dermatitis achieving Investigator’s Global Assessment 0/1 and their mean percentage of body surface area affected in the long-term safety period.46
These data suggest that ruxolitinib cream may delay or prevent the need for systemic therapy in a subset of patients with moderate AD (IGA=3, BSA ≥10% and EASI ≥16 at baseline)
*The VC period included up to Week 8, and the LTS period included Weeks 8–52. Data for Week 8 are from the
VC period.
AD: atopic dermatitis; BSA: body surface area; EASI: Eczema Area and Severity Index; IGA: Investigator’s Global
Assessment; LTS: long-term safety; RUX: ruxolitinib cream; VC: vehicle-controlled.
Regarding the safety profile, most adverse events were mild-to-moderate, with no marked difference between the groups. The most common treatment-emergent adverse events were upper respiratory tract infection and nasopharyngitis. Local reactions, such as burning and itching, were <1% and higher in patients treated with vehicle. Approximately 2% of patients discontinued treatment due to adverse events or serious adverse events, and in most cases, these were not considered related to the drug under the investigator period.
Itch is the most critical symptom to address in patients with moderate AD, and Carrascosa presented data from other studies, the results of which were highlighted as essential for shared decision-making.
Ruxolitinib Cream in Participants with Facial or Neck Atopic Dermatitis Involvement47
In this small, double-blind, vehicle-controlled, Phase 2 study of patients with facial and neck AD (N=77),47 patients were randomised to receive 1.5% ruxolitinib cream (n=54) or vehicle cream (n=23). After 4 weeks of continuous therapy, more than 40% of patients who applied ruxolitinib cream (n=48) compared with vehicle (n=18) saw improvements for facial and neck IGA-TS (IGA 0/1 with ≥2-point improvement from baseline) and achieved head and neck EASI-75 (37.0%; 95% CI: 24.3–51.3% versus 17.4%; 95% CI: 5.0–38.8%; p=0.091).48 Improvements in patient-oriented eczema measure (POEM) scores from baseline were observed and maintained from Week 2 (mean change from baseline: −10.4 versus −3.4) through Week 4 (–11.1 versus −3.7). When patients in the vehicle group switched to ruxolitinib cream at Week 4, similar improvements were observed at Week 8 (–11.1) in those patients randomised to ruxolitinib cream (–11.2).48
Evaluation of the Effect of Ruxolitinib Cream on Itch in Participants with Atopic Dermatitis (SCRATCH-AD)49
The Phase 2, open-label study SCRATCH-AD49 investigated the short-term clinical benefits of this topical agent. Participants with AD (N=46) who applied ruxolitinib cream 1.5% experienced fast and considerable improvement in itch, which was sustained through 28 days of treatment. Itch reduction occurred from Day 1, as early as 15 minutes, and maximum reduction was observed at 4 hours after application of the cream. Post-treatment, patients experienced an improvement of >2 points and the effect continued through 12 hours. Mean change from baseline in modified Peak Pruritus NRS (PP-NRS) was –2.3 (after 15 minutes), peaking at –4.2 (at 4 hours) and –3.1 (at 12 hours).50
Evaluation of the Efficacy and Safety Study of Ruxolitinib Cream in Adults with Moderate Atopic Dermatitis (TRuE-AD4)51
The TRuE-AD4 study has been designed to establish the efficacy of ruxolitinib cream in participants with moderate AD who had an inadequate response to, are intolerant to, or are contraindicated to TCSs and TCIs.51 Patients will be randomised (2:1; N=225 [expected]) to receive ruxolitinib cream 1.5% twice daily (n=150) or vehicle twice daily (n=75) for up to 8 weeks. After this period, patients with no additional safety concerns will continue, and those who are not able to achieve at least EASI-50 will be switched to an escape arm and open-label ruxolitinib 1.5% twice daily as needed until the end of the study at Week 24.
Co-primary endpoints for the study are the proportion of participants with EASI-75 from baseline at Week 8 and the proportion of participants with IGA-TS at Week 8. Key secondary objectives will include an evaluation of the impact of ruxolitinib on patients’ QoL.
Key Takeaways and Concluding Remarks
Moderate AD covers a wide patient spectrum and should be defined using multiple clinical factors, including BSA, assessment scores, impact on QoL, and affected anatomical regions.9,10
Older topical agents like steroids may assist in short-term acute flare management but are less suitable for ongoing control in moderate cases, especially with extensive disease.9 Newer topical treatments, such as ruxolitinib cream, indicate efficacy for rapid itch relief and sustained improvement, for both acute flares and long-term disease maintenance.9 Effective topicals may reduce the need for systemic therapies in moderate AD, provided they are both efficacious and well-tolerated.9,10,11
Moderate-to-severe AD imposes substantial individual and socioeconomic burdens, with persistent symptoms and reduced QoL remaining common due to inconsistent definitions and care fragmentation.2,32-34 Best management includes shared decision-making, considering disease severity, QoL impact, treatment responses, and risk of relapse.1,9,52-55 Ruxolitinib cream provides a novel topical option, and ongoing studies may further define its role.29,50
EU/AD/NP/25/0008
October 2025






