Abstract
Hepatocellular carcinoma (HCC) is the sixth leading cause of cancer-related mortality worldwide. Despite the availability of therapeutic options such as surgical resection, radiofrequency ablation, molecular-targeted agents, and liver transplantation, HCC shows a poor prognosis and limited responsiveness to conventional treatments. The tumour immune microenvironment (TME) influences key processes in HCC, including selection pressure on tumour cells, immune evasion, tumour evolution, treatment resistance, and recurrence. Among immune components within the TME, T cells, dominant among tumour-infiltrating lymphocytes (TIL), exert both suppressive and promotive effects on tumour growth. Thus, T cell-mediated immune responses are fundamental to cancer surveillance and elimination. Research highlights the crucial role of TILs in HCC prognosis, pathogenesis, and immunotherapy. Subpopulations such as Foxp3+ regulatory T cells, CD8+ cytotoxic T cells, and CD3+/CD4+ helper T cells show complex and often contrasting roles. However, the TME often induces T cell exhaustion or dysfunction, facilitating tumour progression and immune evasion. Understanding immune dysregulation is vital for improving anti-tumour immunity and refining T cell function. This review examines TIL subpopulation roles in HCC, emphasising their plasticity and therapeutic relevance. It also covers emerging T cell-based immunotherapies, especially TIL-based adoptive transfer and CAR-T cell therapy, both showing promise in preclinical and early clinical trials. These novel approaches offer new hope for enhancing immune-driven tumour eradication and improving HCC outcomes.
Key Points
1.Hepatocellular carcinoma remains a leading cause of cancer related mortality worldwide, and its immunosuppressive tumour microenvironment limits the efficacy of conventional treatments, highlighting the need for advanced immune based therapeutic strategies.2. This narrative review explores recent advances in T cell-based immunotherapies, including tumour-infiltrating lymphocyte and chimeric antigen receptor-T cell approaches, and their emerging role in treating hepatocellular carcinoma.
3. Integrating chimeric antigen receptor-T or tumour-infiltrating lymphocyte therapies with immune checkpoint blockade and microenvironment modulation may enhance antitumour efficacy, offering new personalised and combinatorial treatment avenues for patients with advanced hepatocellular carcinoma.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth leading cause of cancer-related mortality worldwide.1 Despite therapeutic modalities such as surgical resection, radiofrequency ablation, molecular-targeted agents, and liver transplantation being available, HCC is frequently associated with a poor prognosis and limited responsiveness to conventional interventions.2 Therapeutic modalities vary based on the tumour characteristics, hepatic activity, and physiological condition of the patients.3 To prevent liver parenchyma damage, various mechanisms function to inherently avert undesirable immune responses elicited by contact with microbial antigens and conserved molecular motifs termed danger or pathogen-associated molecular patterns, rendering the liver a predominantly immune-suppressive microenvironment.4 The liver immune microenvironment exhibits operational heterogeneity, characterised by the diverse roles of stromal cells such as liver sinusoidal endothelial cells, hepatic stellate cells, liver resident macrophages (Kupffer cells), and various components of the adaptive immune response, such as CD4+ and CD8+ T lymphocytes and natural killer (NK) cells (Figure 1).5
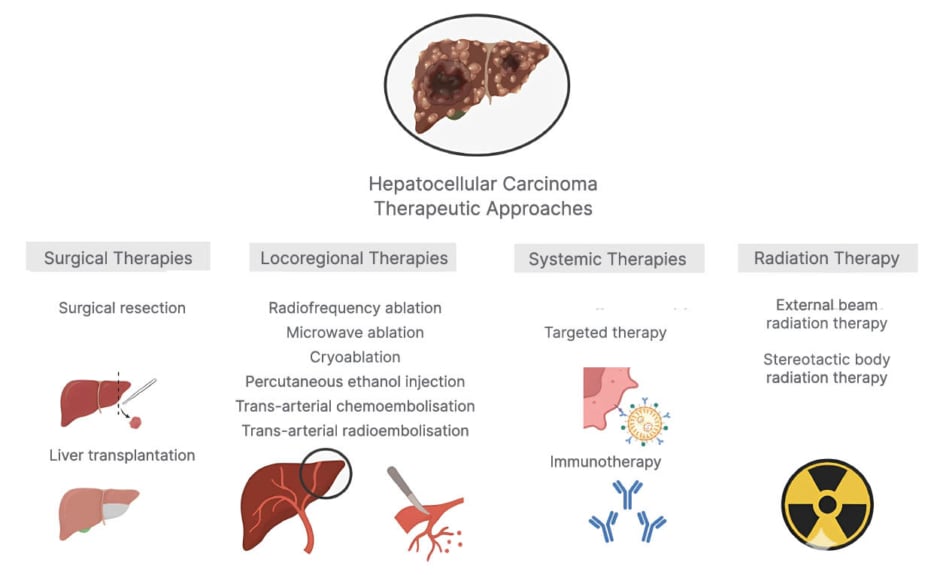
Figure 1: Current therapeutic modalities for hepatocellular carcinoma.
Immunotherapy treatment methods developed in recent years, such as monoclonal antibodies that simultaneously suppress the programmed cell death protein 1 (PD-1) axis and cytotoxic T lymphocyte-associated protein 4 (CTLA-4), could stimulate anti-tumour immunity.6,7 Furthermore, locoregional treatments such as trans-arterial chemoembolisation and ablation produce cell death locally and promote CD8+ cell infiltration into the tumour microenvironment, which supports the use of combination PD-1 blockers.8 In order to circumvent immune escape and trigger anti-tumour responses, autologous T cell transplantation also entails ex vivo activation of mixed T cell/NK cells through cytokine-induced stimulation and reinfusion into the patient. Additionally, ex vivo stimulation of dendritic cells can be used with anti-tumour vaccines against immunodominant peptides of oncofetal proteins to enhance efficient antigen presentation (Figure 2).
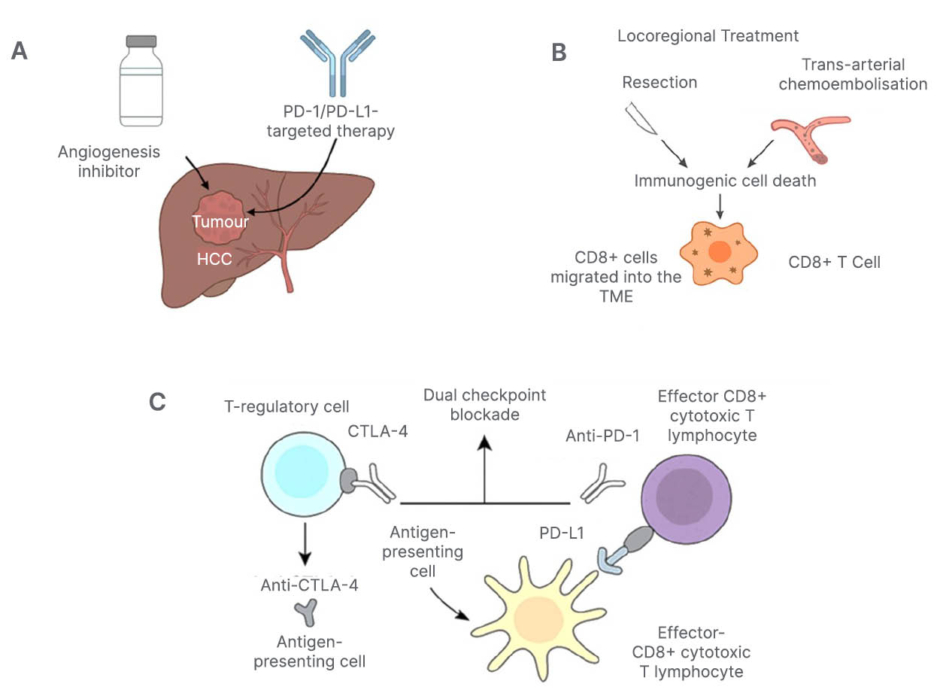
Figure 2: Integrated immunotherapeutic strategies combining checkpoint blockade and locoregional therapies.
A) Schematic depiction of the combination of angiogenesis inhibitors and PD-1/PD-L1-targeted therapy.
B) Locoregional treatments, including resection and trans-arterial chemoembolisation, serve as locoregional
stimulants of immunogenic cell death and promote CD8+ cells to migrate into the tumour microenvironment, hence justifying the use of a combination PD-1 blocker.
C) Concurrent blocking of CTLA-4 and the PD-1 pathway with monoclonal antibodies. The impact of dual checkpoint on T-cell immune reconstitution is shown, with CTL-4 primarily influencing T-regulatory cells and antigen-presenting cells, while PD-1 affects effector CD8+ cytotoxic lymphocytes.CTLA-4: cytotoxic T-lymphocyte-associated protein 4; PD-1: programmed death-1; PD-L1: programmed death-ligand 1; TME: tumour microenvironment.
T cells are an important part of the immune system, and cancer-specific T lymphocytes can experience a gradual decrease in functional activity once subjected to continuous antigenic stimulus. This decrease exhibits several characteristics, such as persistent generation of restrictive receptors, reduced cytokine production, modified metabolic characteristics, inadequate memory re-establishment, and unique transcriptional and epigenomic characteristics, a dynamic phenomenon commonly referred to as T cell exhaustion.9 The present emphasis of immunotherapy is on reversing T cell depletion, seeking to mitigate the detrimental impacts of continuous antigenic induction and offering a pathway for the therapy of HCC. T cells undergo terminal differentiation during cancer progression due to sustained antigenic induction, resulting in T cell exhaustion.10 Naive CD8+ T cells can differentiate into various degrees of exhaustion, characterised by diminishing working and proliferative abilities, which can eventually suffer from overstimulation-induced cell death.11,12 A thorough understanding of the procedure and its fundamental processes is essential to successfully preventing T cell exhaustion. It is necessary to relocate T lymphocytes from a defective developmental pathway before they reach terminal exhaustion.13 In recent years, the efficacy of PD-1/L1 targeted therapy using monoclonal antibodies has led to promising outcomes in solid tumours, such as HCC, and prompted growing efforts to focus on immunotherapy to reactivate T cell function for tumour treatment.14
In this light, adoptive cell transfer, like tumour-infiltrating lymphocytes (TIL), and chimeric antigen receptor (CAR)-T cell therapy, not only modulates immune equilibrium within the tumour microenvironment but has also demonstrated significant efficacy in treating various malignancies, thereby heightening clinical researchers’ interest in this area.15 Nonetheless, the use of both adoptive cell treatment and immune-targeted therapies will bring about the challenge of T cell exhaustion.16-18 This study will outline the significant T cell subsets found in HCC and examine existing T cell-based immunotherapies in HCC, focusing on TILs and CAR-T cell-based therapies.
SUBTYPES OF TUMOUR-INFILTRATING T CELLS IN HEPATOCELLULAR CARCINOMA
CD8+ Cytotoxic T Lymphocytes
CD8+ cytotoxic T lymphocytes are crucial for mediating antigen-specific cytotoxicity through tumour-associated antigens presented by antigen-presenting cells, including dendritic cells. The cytotoxic T cell receptor (TCR) binds to the tumour antigen presented by major histocompatibility complex class I, and becomes mature. Then CD8+ mature T lymphocytes induce cytotoxicity by immediate destruction of neoplastic cells and the release of enzymes along with destructive mediators, including perforin/granzyme B, interferon gamma (IFNγ), and TNFα.19 Perforin disrupts the integrity of the tumour cell membrane, while granzyme B triggers apoptosis through caspase activation, via interaction with Fas ligand expressed by CD8+ T lymphocytes with Fas receptors on cancerous cells, and initiates the caspase signalling axis. TNF induces inflammatory responses and apoptosis by activating caspase-mediated signalling pathways in tumour cells.20-22
Clinically, considerably higher levels of CD8+ T cells are found in HCC, particularly within or around neoplastic tissue, identifying them as key components of tumour-infiltrating lymphocytes for monitoring HCC development.23 Cytotoxic lymphocytes, along with an elevated CD4+/CD8+ ratio, are correlated with a reduced risk of relapse following hepatic transplantation.24 The effectiveness of anti-tumour immunological response requires not only antigen presentation and T cell priming, but also efficient infiltration and persistence of effector T cells within the tumour parenchyma. Immune-desert phenotype, immune-excluded phenotype, and inflamed phenotype are three tumour penetration types based on pathology investigations. The amount and quality of invading T cells both enhance anti-cancer effectiveness.25,26
Previous research has proved that the proportion of infiltrating CD8+ T cells is considerably lower in HCC tissues compared to non-cancerous liver tissue.27 Furthermore, the immunosuppressive function of regulatory T cells (Tregs) and CD8+ T cells within the tumour microenvironment often exhibits functional defects, which are evident through exhaustion and immune tolerance. This pathologic state entails reduced cytotoxic activity and elevated expression of inhibitory immune checkpoint receptors, such as PD-1, LAG-3, TIM-3, and CTLA-4. The loss of CD8+ T cells is also promoted by the immunosuppressive activities of Tregs, which together compromise the effectiveness of the anti-tumour immune response. Inside the tumour microenvironment, CTLs may undergo activation-induced cell death, a process which is induced by chronic antigen stimulation and inflammatory stress.12 Empirical evidence based on murine models of HCC has demonstrated increased rates of apoptosis in intratumoral CD8+ T cells, thus substantiating the belief that the tumour microenvironment is actively engaged in facilitating T cell dysfunction and consequent loss of cells. Collectively, these findings demonstrate that CD8+ T cells are crucial for the mediation of anti-tumour immunity; their quantity, quality, and longevity in the TME ultimately determine their therapeutic potential in HCC.28-30
Regulatory T Cells
Regulatory T cells are a distinct subset of CD4+ T lymphocytes that play a critical role in the induction of immune tolerance and are critical in maintaining immune homeostasis. In the tumour microenvironment of HCC, the existence of Tregs facilitates immune evasion by cancer cells by suppressing effective anti-tumour immune responses.31 In tumour tissues, Tregs are generally divided into two major subsets: natural Tregs and inducible Tregs. Natural Tregs with the CD4+CD25+FOXP3+ phenotype are the prominent subset and play a critical role in the maintenance of immunological homeostasis through the regulation of peripheral inflammation.32,33 These cells possess the capacity to exert immune suppressive function in the tumour via the Fas-FasL pathway, and importantly, induce apoptosis in natural killer cells and CD8+ cytotoxic T lymphocytes in a granzyme B/perforin-dependent process.34,35 Inducible Tregs, that may or may not express FOXP3, perform immunosuppressive activities by mainly secreting anti-inflammatory cytokines IL-10 and transforming growth factor-TGF-β (TGF-β), and by producing adenosine. All these events serve to inhibit the activation and functionality of effector B and T lymphocytes following contact with an antigen. While Tregs are essential under normal circumstances to prevent autoimmunity and regulate inflammatory responses, their inappropriate expansion and activity in the TME of HCC facilitate tumour immune evasion.36 The immunosuppressive role of Tregs operates via multiple mechanisms, including the release of inhibitory cytokines, direct elimination of effector T cells through granzyme and perforin pathways, and the alteration of dendritic cell differentiation and their antigen-presenting ability.37 The mechanism above entails the regulatory signalling mediated by inhibitory receptors like CTLA-4 and LAG-3.38 Surprisingly, the concurrent activation of both the TCR and IL-2 receptor is central to the survival of Tregs and their immunosuppressive functions.39 In HCC, Tregs predominantly undermine anti-tumour immunity through the inhibition of CD8+ T cell activities. Mechanistic studies have also shown that Tregs derived from HCC can downregulate the co-stimulatory molecules CD80 and CD86 on DCs in a CTLA-4-dependent manner, dampening antigen presentation and inhibiting effector T cellresponses further.40,41
Clinical observations have revealed significantly increased percentages of CD4+CD25+ Tregs in peripheral blood as well as liver tissues of patients with HCC compared to healthy controls. Such an increase is linked with disease progression and poor prognosis. Additionally, a high intratumoural Treg-to-CTL ratio has been identified as a prognostic factor, where higher ratios are correlated with poorer overall survival and disease-free survival.42 In a study of 19 patients with advanced HCC, lower frequencies of PD-1+ and FOXP3+ Tregs were correlated with increased survival after sorafenib treatment, suggesting their utility as predictive biomarkers. Additionally, evidence exists that Treg accumulation can be causally responsible for HCC recurrence following liver transplantation through a CXCL10–CXCR3 chemokine axis. In preclinical models, depletion of Tregs using monoclonal antibodies against CD25 has been effective at preventing HCC progression, highlighting a potential therapeutic avenue thatinvolves reprogramming the immunosuppressive TME.43,44
Th1 and Th2 Helper T Cells
T helper cells are a heterogeneous population of CD4+ T lymphocytes that orchestrate immune responses by secreting various cytokine profiles.45,46 Th1 and Th2 cells are two principal subsets among them, which have different cytokine secretion patterns and immunological roles. Th1 cells are mainly responsible for producing IFNγ, IL-2, IL-22, and TNFβ, whereas Th2 cells are characterised by the secretion of IL-4, IL-5, IL-9, IL-10, and IL-13.47,48 These polarised subsets play complementary but often opposite functions: Th1 cells play a central role in inducing cell-mediated immunity and delayed-type hypersensitivity, whereas Th2 cells induce humoral immune responses and are involved in regulating the production of antibodies.49-51 In HCC, the functional distinction between Th1 and Th2 cells is crucial for determining the nature of the TME and the course of the disease. Th1 cells, on recognising peptide-major histocompatibility complex class II complexes and interacting with co-stimulatory molecules, produce pro-inflammatory cytokines like IFNγ, which are accountable for the recruitment and activation of CD8+ CTLs in tumour tissue.52,53 This Th1-induced immune activation enables productive anti-tumour immunity. The shift from Th1-dominant to Th2-dominant cytokine milieu is frequently observed in HCC and signifies the setup of an immunosuppressive TME.54,55 While Th1-associated cytokines IL-1α, IL-1β, IL-2, and IFNγ are typically associated with good clinical outcomes and improved prognosis, overexpression of Th2 cytokines IL-4, IL-5, and IL-10 is correlated with more invasive tumour behaviour and poor prognosis. This Th1-to-Th2 transition has been regarded as a hallmark of immune evasion in HCC.56,57 Moreover, increased circulating Th2 cells have been associated with progressed HCC and decreased efficacy of treatment options available. Research demonstrated that individuals with lower Treg levels at baseline exhibit an improved Th1/Th2 ratio prior to trans-arterial chemoembolisation, a widely applied locoregional therapy for HCC. Importantly, a higher Th1/Th2 ratio was linked to more intense anti-tumour immunity and prolonged survival rates. Although cancer vaccines targeting helper T cells have reported promising activity in a number of malignancies, the specific contribution of Th1 responses to promoting effector T cell activation in HCC remains ill-defined. Further research is required to establish the therapeutic utility of adjusting the Th1/Th2 balance as a strategy to reverse immunosuppression and enhance anti-tumour immunity in HCC.58
CRITICAL SIGNALLING PATHWAYS MODULATING TUMOUR-INFILTRATING LYMPHOCYTES IN HEPATOCELLULAR CARCINOMA
TGFβ Signalling
The TGFβ family includes TGFβs, activins, inhibins, bone morphogenetic proteins, and growth and differentiation factors.59,60 The initiation of this axis begins with the attachment of ligands to the extracellular domains of TGFβ Type I and Type II receptors (TβRI and TβRII), stimulating both suppressor of mothers against decapentaplegic homolog (SMAD)-reliant and SMAD-non-reliant axis to elicit the downstream pathway. The TGFβ axis is implicated in almost every phase of tumorigenesis in HCC.61 The TME exhibited elevated levels of TGFβ in both cancer cells and several immunological cells. In the first phase of HCC, TGFβ inhibited the growth of pre-malignant hepatocytes. However, in the developed phase of HCC, it facilitated cancer growth by modulating immunological cells, including Tregs, CTLs, TAMs, and NKs.62 TGFβ may up-regulate FOXP3 in CD4+CD25-naïve T cells by promoting the SMAD-reliant axis, facilitating the development of Tregs. Tregs from peripheral blood penetrated HCC tumour tissues, thereby inhibiting the therapeutic efficacy of CTLs. Moreover, HCC cells demonstrate elevated levels of TGFβ, thus underlying the upregulation of PD-1 levels on CD8+ CTLs. CTLs subsequently attach to PD-L1 on malignant cells and antigen-presenting cells, resulting in CTL exhaustion.63,64 TGFβ activation may regulate the innate immune system by inhibiting NK cells directly and facilitating M2 macrophage polarisation to promote immune evasion. TGFβ signalling pathways were extensively implicated in the control of TILs, ultimately facilitating HCC progression.61,62
JAK/STAT3 Signalling
The STAT protein family has seven members, including STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6. STAT3 has been the subject of several studies concerning HCC management. It was believed to be triggered swiftly and temporarily in normal cells by various mediators, including IL-6/IL-10, IFNs, and growth factors like vascular endothelial growth factor. Nonetheless, it was consistently and aberrantly activated in neoplastic cells.65-68 Recent research indicated that phosphorylated STAT3 was present in approximately 60% of HCC samples, correlating with a worse prognosis. The STAT3 axis primarily promotes tumorigenesis by modulating dendritic cells and developing tumour-associated macrophages, NK cells, and Tregs.68,69 In DCs, IL-6 released through HCC cells and hepatic carcinoma-associated fibroblasts can attach to the IL-6 receptor on DCs, activating JAK and initiating the downstream phosphorylation STAT3 axis, which inhibits T cell growth and enhances Tregs expansion.70 Furthermore, the stimulation of STAT3 has been shown to suppress the activating receptor NKG2D on NK cells and its associated ligands, major histocompatibility complex class I-related protein A/B on malignant cells, hence obstructing NK cell stimulation and leading to impaired immune surveillance in HCC.71 Inhibition of STAT3 signalling in HCC may reactivate NK cells to perform anti-tumour functions via modifying cytokine levels in the TME, specifically by decreasing IL-10 levels. Additionally, the IL-6-STAT3 axis promoted tumorigenesis by diminishing M1 macrophage polarisation and enhancing M2 differentiation of TAM. STAT3 activation affected many immunological cells, contributing to the progression of HCC.72,73
Wnt/β-Catenin Signalling
The Wnt signalling pathway becomes active when Wnt proteins engage with their specific membrane-bound receptors, initiating a cascade of intracellular events. One key player in this pathway is β-catenin, a protein usually associated with cell-to-cell adhesion or found in the cytoplasm. Upon pathway activation, β-catenin accumulates and translocates to the nucleus, where it influences gene expression. In HCC, persistent activation of the Wnt/β-catenin axis has been frequently observed and is linked to immune evasion. This activation impairs the infiltration and function of tumour-infiltrating lymphocytes, especially by reducing dendritic cell presence, which plays a crucial role in antigen presentation and T cell priming. Consequently, this weakens the recruitment and cytotoxic function of CD8+ T cells. Chronic stimulation of this axis also contributes to cytotoxic T lymphocyte dysfunction, ultimately fostering an immunosuppressive environment favourable to tumour progression. Overall, the aberrant Wnt/β-catenin signalling in HCC is a critical factor in disrupting immune surveillance and supporting tumour immune escape mechanisms (Table 1).44,74-78
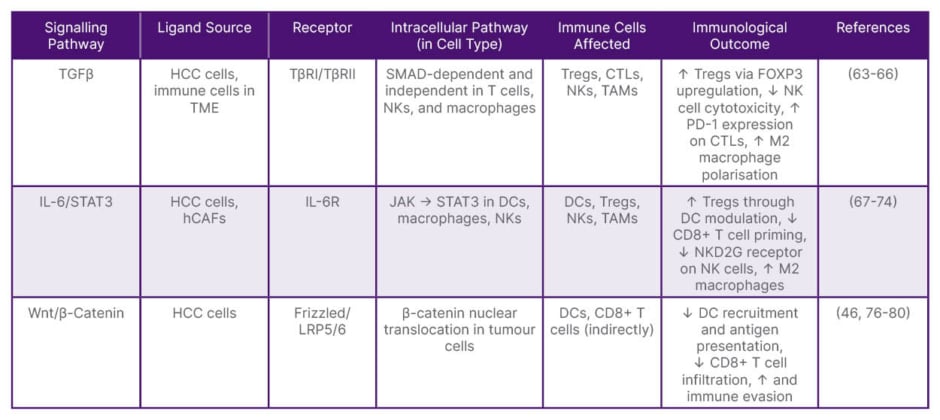
Table 1: Critical immunoregulatory signalling pathways affecting tumour-infiltrating lymphocytes in hepatocellular carcinoma.
CTL: cytotoxic T lymphocyte; DC: dendritic cell; hCAF: hepatic cancer-associated fibroblast; HCC: hepatocellular carcinoma; NK: natural killer; PD-1: programmed death-1; TAM: tumour-associated macrophage; TGFβ: transforming growth factor-β; TME: tumour microenvironment; Tregs: regulatory T cells.
TUMOUR-INFILTRATING LYMPHOCYTE THERAPY IN HEPATOCELLULAR CARCINOMA
TIL therapy is a promising immunotherapeutic approach for HCC, leveraging the patient’s own tumour-reactive T cells. These lymphocytes are isolated from the tumour, expanded in vivo, often with IL-2, and reintroduced after lymphodepletion to enhance their persistence and efficacy.79-81 This strategy boosts the body’s immune response by restoring and amplifying tumour-specific T cell activity. However, challenges remain, including difficulty in isolating sufficient TILs from the liver’s immunosuppressive environment and ensuring effective tumour antigen recognition. Current research aims to optimise expansion techniques, tailor conditioning regimens, and overcome immune resistance. Continued refinement is essential to establish TIL therapy as a reliable treatment option for HCC.82-86
RECENT ADVANCES AND CLINICAL APPLICATIONS
Recent advances in tumour-infiltrating lymphocyte therapy have positioned it as a promising immunotherapeutic option for HCC. Early-phase clinical trials, such as the Phase I trial of BST02, a TIL-derived product, have demonstrated encouraging outcomes in patients with advanced HCC. Notably, combining TILs with immune checkpoint inhibitors, particularly PD-1/PD-L1 blockade, appears to overcome the immunosuppressive liver microenvironment, leading to more durable antitumor responses compared to TIL monotherapy.87-90 To further improve efficacy, researchers have employed adjunct strategies such as IL-2 to enhance TIL proliferation and function. Pre-treatment lymphodepletion with agents like cyclophosphamide has also shown benefit by facilitating TIL engraftment and expansion. However, the tumour microenvironment, characterised by high PD-L1 expression and suppressive cytokines, continues to limit therapeutic success. To address this, novel approaches, including oncolytic virotherapy and lymphodepleting regimens, are being evaluated for their synergistic potential to reprogram the TME.91,92 In parallel, TIL engineering strategies such as CAR expression have shown promise in preclinical models, offering increased tumour specificity. Phase II trials report that combination regimens remain safe and tolerable, even in patients with chronic hepatitis B, although tumour regression is modest in this subgroup. Overall, the future of TIL therapy in HCC lies in personalised approaches informed by immune profiling and the strategic integration of combination therapies. These advancements may ultimately establish TILs as a foundational component of immunotherapy in livercancer (Table 2).25,93,94
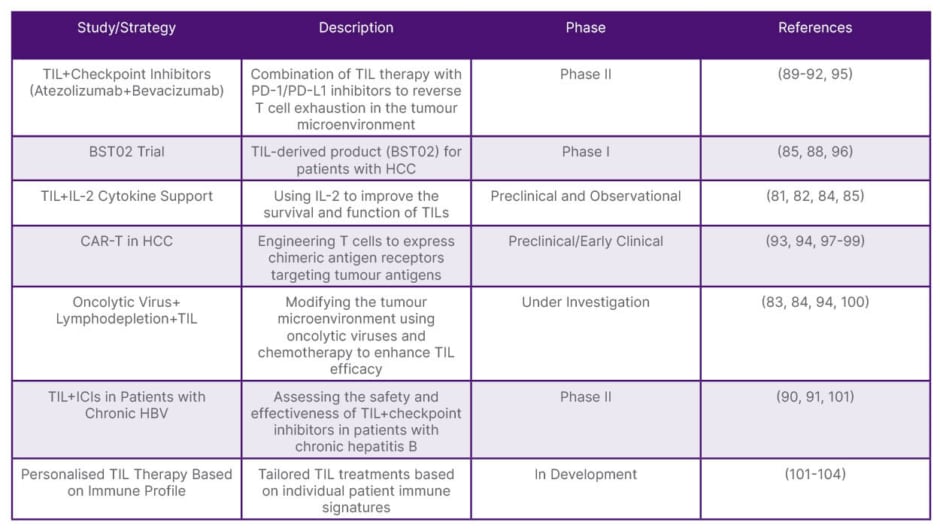
Table 2: Recent studies on tumour-infiltrating lymphocytes in hepatocellular carcinoma.
HBV: hepatitis B virus; HCC: hepatocellular carcinoma; PD-1: programmed death-1; PD-L1: programmed death-ligand 1; TIL: tumour-infiltrating lymphocytes.
BIOMARKERS AND PREDICTIVE INDICATORS
Biomarker identification is pivotal for optimising patient selection and improving the therapeutic efficacy of TIL therapy in HCC. Accumulating evidence suggests that a high frequency of CD8+ TILs within tumour tissues is associated with superior clinical outcomes, particularly when TIL therapy is combined with immune checkpoint inhibitors. Tumours enriched in CD8+ T cells may demonstrate enhanced immune responsiveness, making them more amenable to TIL-based interventions. Nonetheless, the predictive roles of additional immune biomarkers, such as PD-L1 expression and TCR diversity, remain under active investigation. Furthermore, both liver functional status and HCC staging are important considerations in determining patient eligibility. Individuals with preserved liver function and early-stage disease are more likely to benefit from adoptive TIL therapy.95, 101 Recent clinical studies have yielded variable results regarding its therapeutic impact. A Phase I trial published in 2022 demonstrated the technical feasibility of TIL isolation, expansion, and reinfusion in patients with HCC; however, clinical responses were limited, particularly in heavily pre-treated individuals.102 More recent investigations have evaluated the combination of TIL therapy with PD-1 inhibitors, showing promising early efficacy, especially in tumours with high PD-L1 expression.96,103 These findings underscore the need for improved patient stratification strategies and potentially the incorporation of additional combination therapies to enhance response. Moreover, TME-induced immunosuppression continues to be a major barrier to TIL functionality in HCC. Observational studies confirm that overcoming the TME’s suppressive effects is critical for maximising TIL efficacy, aligning with previous research emphasising the central role of the TME in modulating immune responses in liver cancer.105,106
ADVANCES IN CAR-T CELL THERAPY FOR HEPATOCELLULAR CARCINOMA
CAR-T cell therapy is being actively explored for HCC, with recent innovations addressing key challenges, such as immune evasion, tumour heterogeneity, and a suppressive microenvironment. Glypican-3 (GPC3), highly expressed in HCC and rarely in normal tissues, is a leading CAR-T target. Early clinical results show anti-tumour activity, though efficacy is limited by immunosuppression (Figure 3). To counter this, GPC3-CAR-T cells are engineered to release PD-1 or TGFβ inhibitors, enhancing persistence and function. Co-administering checkpoint inhibitors (e.g., anti-PD-1) also helps reduce T cell exhaustion.97,104,107 Tandem CARs (TanCARs), recognising GPC3 and AFP, boost tumour detection and reduce immune escape. SynNotch CARs use a two-step activation model, first sensing one antigen, then triggering CAR expression via another, ensuring tumour specificity and minimising off-target effects. Both methods improve tumour targeting in HCC models.44,98,108 HCC’s immunosuppressive environment hinders CAR-T activity. New CAR-Ts are modified to secrete cytokines like IL-15, supporting T cell survival. Targeting vascular endothelial growth factor improves infiltration, while combining with TGFβ inhibitors reprogrammes the microenvironment to be more immune-permissive.108 Additionally, Universal CARs employ a modular ‘lock-and-key’ system, allowing redirection to multiple tumour antigens. This approach addresses tumour heterogeneity and lowers production costs, improving feasibility for personalised treatments.99,109 CAR-T efficacy is improved by blocking inhibitory receptors such as PD-1, LAG-3, and TIM-3. Inhibiting CD39, a driver of adenosine-mediated suppression, restores function in HCC. Combining CAR-T with anti-LAG-3 antibodies further revives exhausted T cells.110-112 Engineered cytokine receptors like 4/7 ICR convert suppressive IL-4 signals into stimulatory IL-7-like effects. Advanced 4/21 ICR CAR-T cells adopt a Th17-like phenotype under IL-4, enhancing anti-tumour activity and persistence in HCC.113,114 To prevent recurrence, CAR-T cells are engineered to produce IL-7 or IL-21, promoting memory cell development. Small-molecule agents like T-lymphokine-activated killer cell-originated protein kinase inhibitors and CAR signalling modifications improve survival. Combining CAR-T with chemo, radiation, or oncolytic viruses further boosts immune response and tumourantigen visibility.115,116
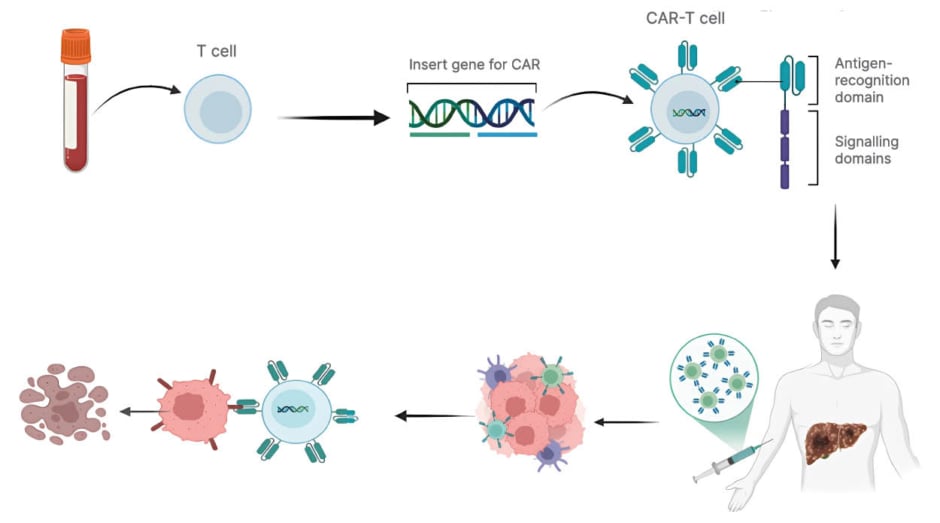
Figure 3: Schematic representation of CAR-T cell therapy in liver cancer.
Peripheral blood T cells are isolated and genetically engineered to express CAR. The modified CAR-T cells are expanded and re-infused into the patient, where they recognise and kill tumour cells in the liver through antigen-
specific cytotoxicity.
CAR: chimeric antigen receptor.
CONCLUSION
Recent evidence has revealed that cancer progression is not only driven by local immune and molecular alterations but also by systemic neuroendocrine regulation. Tumours can hijack neuroendocrine signalling pathways to manipulate host homeostasis, thereby creating conditions favourable for their growth and immune evasion. Through the release of stress-related hormones and neuropeptides, the tumour can modulate immune cell activity, suppress cytotoxic CD8+ T lymphocytes, and promote Treg expansion within the tumour microenvironment. This neuroendocrine–immune interaction leads to impaired anti-tumour immunity and contributes to T cell exhaustion, a key obstacle to effective immunotherapy. Understanding how HCC exploits neuroendocrine mechanisms to suppress immune surveillance may open new avenues for combination therapies that integrate immunomodulation with neuroendocrine targeting to restore both immune balance and systemic homeostasis.100 Recent advances in immunotherapy have rekindled hope for HCC treatment, with T cell exhaustion receiving more attention. This pathological condition is one of the driving forces for HCC development, but its causative mechanisms are only half understood and require more clinical investigations. Clarification of the molecular mechanisms of T cell exhaustion is essential for unravelling the immune microenvironment of HCC and developing new therapeutic interventions. Both TILs and CAR-T cells possess double-edged roles, both fighting cancer and potentially supporting tumour growth, via a complex network of signalling interactions within the tumour microenvironment. Further research is necessary to unveil the occult functions and mechanisms of TILs, particularly how they mould and are moulded by the evolving HCC environment. Longitudinal studies of the functions of TILs and CAR-T cells in HCC development will provide additional information on immune-tumour interactions and guide the development of next-generation immunotherapeutic approaches. As a narrative review, this study is limited by the lack of a systematic search strategy, which may introduce selection bias. Additionally, due to the rapidly evolving nature of immunotherapy research, some recent data may not have been captured at the time of writing. Finally, many referenced findings originate from early-phase or preclinical studies, which limits the strength and generalisability of the conclusions.







