Shellfish is one of the most common food allergens worldwide, causing frequent anaphylaxis in both paediatric and adult populations.1 Despite the identification of tropomyosin as the major cross-reactive shellfish allergen for over two decades,2 strict avoidance and epinephrine injection remain the predominate preventive and therapeutic recommendations. To provide an allergen-specific immunotherapeutic option, the authors constructed a hypoallergen of shrimp tropomyosin, namely MEM49, by site-directed mutagenesis within the major IgE-binding epitopes.3 The prophylactic and therapeutic
values of a MEM49-encoding DNA vaccine (pMEM49) constructed with the mammalian expression vector pCI-Neo were further demonstrated using a well-established BALB/c mouse model of shrimp tropomyosin-induced hypersensitivity.4-6 Specifically, pMEM49 immunisation resulted in the down-modulation of allergen-specific IgE synthesis, intestinal expression of Th2 cytokines, and infiltration of inflammatory effector cells accompanied with increased regulatory T cell frequency.
One of the most critical outcomes of pMEM49 vaccination is the suppression of tropomyosin-specific IgE synthesis. The authors hypothesise that such suppression can be achieved by restricting Th2 cell signalling via the induction of regulatory immune cells. In an attempt to explore the potential pathways leading to the induction of regulatory cells by pMEM49 immunisation, RNA-sequencing was performed using the Ion Torrent Proton Platform (BGI, Shenzhen, China) with ileum samples from the negative control, positive control (PBS), prophylactic (P-MEM49), and therapeutic (T-MEM49) groups (Gene Expression Omnibus accession number: GSE86840). Differential gene expression profiles compared between each pair of animal groups were correlated to the immunological signatures available on ImmuneSigDB, PaGenBase, and the authors’ in-house generated database by gene set enrichment analysis (GSEA),7 to identify candidate pathways and key genes involved in the immuno-regulation by pMEM49.
The authors first identified significant enrichment of genes associated with the induction of tolerogenic dendritic cells (tDC), with gene signatures of P-MEM49 and T-MEM49 consistent with the RNA profile of tDC activated by vitamin D3. This includes heightened metabolic processes such as glycolysis and oxidative phosphorylation through stimulating the PI3K/AKT/mTOR pathway (Figure 1). pMEM49 also induced overexpression of IDO1, which was shown to maintain oral tolerance by supporting the TGF-β synthesis8 that was also upregulated in the pMEM49-immunised animals. The authors thus speculate that this specific pathway is important to the inhibition of Th2 responses.
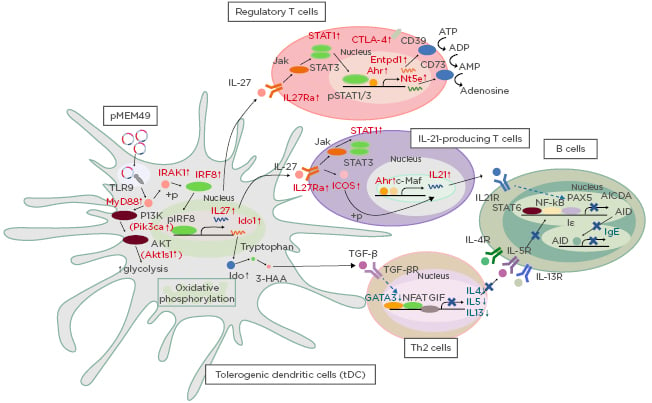
Figure 1: Proposed model for the mechanistic action of pMEM49 immunisation in inducing tDC and Treg cells that repress IgE synthesis in B cells.
tDC: tolerogenic dendritic cell; Th: T helper cell; Treg: regulatory T cell.
In addition to gene sets related to tDC induction, there was also significant enrichment of gene sets concordant to regulatory T cell activities, such as upregulation of IL27, IL27R, CD39, CD73, and CTLA-4. In addition, IL21 also represented as a core enriched gene, which is well-illustrated as a key cytokine for the repression of IgE synthesis. This is achieved by inhibiting germ line transcription, partly through the diminishing of PAX5 expression.9 In fact, PAX5 downregulation was validated in both P-MEM49 and T-MEM49 RNA samples. Notably, tDC-produced IL-27 is a potent transactivator of IL-21 production through induction of c-Maf and ICOS expression.10 More strikingly, these key genes involved in this IL27-IL21 axis were all upregulated in our P-MEM49 and T-MEM49 samples compared to the
control groups.
In summary, these data suggest the potential of pMEM49 in activating tDC that synthesise IL-27 to orchestrate the generation of IL-21-producing T cells. Together with the action of TGF-β, which limits the Th2 response, IgE synthesis is restricted via a PAX5-dependent pathway. Although the present study is limited by the lack of cell-specific analysis, the data provided herein sheds light on candidate molecules and immunological pathways involved in this platform of DNA vaccine-based modality.






