Allergic rhinoconjunctivitis is a Th2-type inflammatory disease of the nasal mucosa and the conjunctiva, mediated by immunoglobulin (Ig)E antibodies. The prevalence of allergic rhinitis in Europe is ˜25.0%,1 ranging from 17.0% (Italy) to 28.5% (Belgium).2 Inhaled allergens are an important factor in morbidity, and they may severely affect quality of life. Over recent decades, a matter of particular concern has been Ambrosia (ragweed) pollen, its presence in the air being determined by the plants dispersing it and the meteorological processes that alter pollen release, dissemination, transport, or deposition on surfaces.3 The aim of this study was to determine the impact of ragweed pollen on allergic patients from western Romania.
A total of 97 subjects were recruited prospectively between August and November 2013, in an observational cross-sectional study. Of the 97 individuals, 84 with positive skin prick test (SPT) to ragweed pollen extract and 13 negative controls were included. Patient evaluation was performed by SPT with a panel of 18 standard inhaled allergens. Specific serum IgE to 176 allergens was determined by using the ImmunoCAP ISAC microarray (Phadia AB, Uppsala, Sweden).
Mean patient age was 31.2±8.9 years, with a mean allergic disease age of 3.62±3.15 years. Of the patients, 40% were male. Patients with a positive SPT to ragweed pollen extract totalled 84. Most patients (73%) had moderate-severe intermittent allergic rhinoconjunctivitis, and 25% of them also had allergic asthma. Out of the 84 patients with a positive SPT to ragweed pollen extract, 90% also had increased specific serum IgE levels to the major ragweed allergen, Amb a 1 (5% Class 1, 35% Class 2, and 45% Class 3) (Figure 1). Among the patients who were tested for multiple allergens by SPT, the majority (78%) were polysensitised. Polysensitised patients were mostly sensitised to other pollens (cereals, Artemisia, birch, and oak), house dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae), and pet dander (dog, cat). The sensitivity and specificity of the ImmunoCAP ISAC microarray (Amb a 1) versus SPT (ragweed pollen extract) was 85.88% (95% confidence interval [CI]: 76.63–92.48%), and 90.91% (95% CI: 58.67–98.49%), respectively. IgE class did not correlate with SPT response class. We investigated potential cross-reactivity between Amb a 1 and other allergens from the same family, from species not present in the local, indigenous flora: Cry j 1 (Japanese cedar) and Cup a 1 (Arizona cypress). Cry j 1 specific IgE were identified in 24% of the Amb a 1 positive patients, as opposed to none of the Amb a 1 negative patients, which was a statistically significant difference (p<0.05). Cup a 1 specific IgE were identified in 8% of the Amb a 1 positive patients, as opposed to none of the Amb a 1 negative patients, which was statistically insignificant (p>0.05) (Figure 2). A moderate correlation was found between the self-reported evaluation score (on a scale 1–10) and a computed symptoms score (total symptoms a patient presents), by using Spearman’s rank correlation coefficient (0.584), which was extremely significant (p<0.0001), meaning that the patients’ estimate of disease severity correlated well with the number of symptoms. All patients with a disease history older than 15 years had developed allergic asthma.
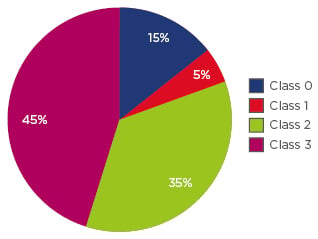
Figure 1: ImmunoCAP ISAC microarray results.
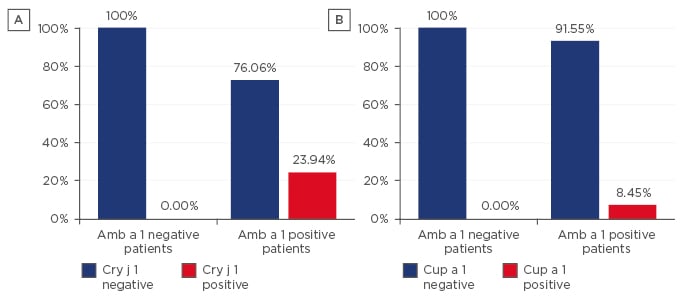
Figure 2: Potential cross-reactivity between Amb a 1 and other allergens from the same family for both Amb a 1 positive and negative patients.
A) Cross-reactivity between Amb a 1 and Cry j 1; B) Cross-reactivity between Amb a 1 and Cup a 1.
There was a small fraction of patients that were allergic to minor ragweed pollen allergens and they were not identified by standard in vitro diagnostic procedures. Component-based diagnosis using microarrays is needed for the selection of the appropriate allergen source for specific immunotherapy. Appropriate therapeutic measures need to be taken in order to prevent progression of allergic inflammation from rhinoconjunctivitis to bronchial asthma.






