BACKGROUND
Eosinophil-derived neurotoxin (EDN), as a biomarker of eosinophilic activity, can be measured in blood matrices such as serum, ethylenediaminetetraacetic acid (EDTA) plasma, and heparin plasma.1 For unclear reasons, current EDN studies aim to use serum, even though the manufacturer’s assay validation identifies it as the least suitable matrix of choice.2,3 Moreover, the same validation data do not include reference values for adults.4 The goal of this study is to establish EDN reference intervals, means, and medians in different blood matrices in adults, and to argue which blood matrix should be measured for EDN.
METHODS
Blood samples were randomly selected from a common hospital population. Collections were included if all three matrices were available: EDTA plasma, Barricor™ lithium-heparin plasma (Becton Dickinson and Company, Franklin Lakes, New Jersey, USA), and serum. Samples were treated according to common routine practice: centrifugation of both serum and lithium-heparin samples at 1,855×g for 10 minutes, and EDTA samples at 1,150×g for 10 minutes. EDN values were measured using the ImmunoCAP™ assay (Thermo Fisher Diagnostics Phadia, Uppsala, Sweden). Values below the lower limit of quantitation (2 μg/L) were fixed at 1.41 μg/L, while those above the upper limit of quantitation (200 μg/L) were diluted five-fold and remeasured. Reference 95% intervals, means, and medians for EDN values were calculated after logarithmic transformation.
RESULTS
The age characteristics of the subjects studied (N=51) were as follows: range 15–78 years, mean age 43.6 years, and median age 35.0 years. These values are shown in Figure 1A. The reference intervals, means, and medians for EDN values were: EDTA plasma 2.1–197, 16.2, and 14.7 μg/L; Barricor™ lithium-heparin plasma 2.7–251, 50.1, and 50.9 μg/L; and serum 2.5–218, 34.7, and 33.2 μg/L, respectively (Figure 1B).
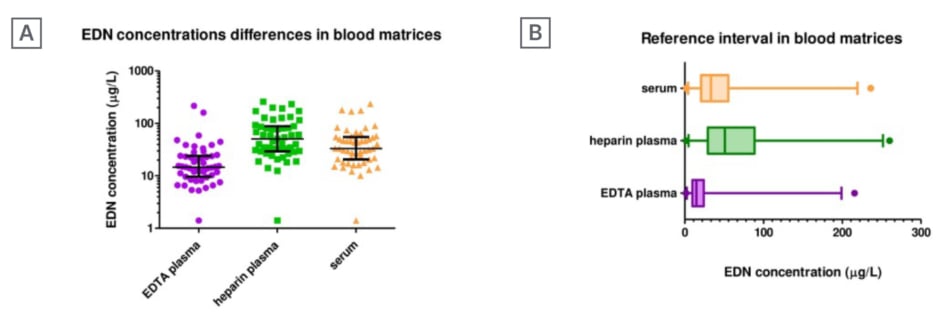
Figure 1: Eosinophil-derived neurotoxin concentrations, in a common hospital population, measured in three
different matrices under matrix-specific pre-analytical conditions.
A) Distribution of data displayed on logarithmic scale. B) Box-and-whiskers plot of the same data, showing the values below the 2.5th percentile and above the 97.5th percentile as individual circles.
EDN: eosinophil-derived neurotoxin; EDTA: ethylenediaminetetraacetic acid.
CONCLUSION
Eosinophils are sensitive to both sample collection and treatment conditions, while the post-collection release of EDN should be avoided.5 Among the tested blood matrices, the one with the lowest reference interval, mean, and median is considered ideal. According to the manufacturer’s validation data, EDTA plasma emerges as the logical matrix of choice, while serum is the least favourable choice. This study, conducted under common routine practice, confirms the choice of EDTA plasma, although the author’s ranking also proposes that Barricor™ lithium-heparin plasma is the least favourable choice. The manufacturer of the ImmunoCAP™ assay suggests that EDTA inhibits enzymatic processes, whereas heparin is supposed to leave the eosinophils in a less activated state compared to serum. The authors claim that the treatment of samples, the activation by the applied centrifugation conditions, and the separation by the Barricor™ system, is critical. Therefore, the EDN value measured in EDTA plasma more reflects the true EDN concentration in blood. The author’s reference intervals are based on a general hospital population, which includes individuals with both EDN-related and unrelated pathologies, and do not represent a purely healthy cohort.




