Abstract
Background: COVID-19, a global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in December 2019 in China and has spread to 210 countries and territories. Since then, it has infected >187.3 million people worldwide, causing >4.0 million deaths, and numbers are continuing to rise. Fever, dry cough, shortness of breath, and pneumonia are the main symptoms of this disease, which does not have any specific antiviral treatment or vaccines to date, and clinical management is mainly symptomatic treatment.
Summary: The global spread of SARS-CoV-2 has necessitated the development of novel therapeutic agents against the virus to stop the pandemic. Drugs targeting viral as well as host factors may have a potential antiviral effect. The development of novel drugs may take years; hence, the best alternative available is to repurpose existing antiviral drugs with a known safety profile in humans. Further, compounds with known in vitro and in vivo efficacy against SARS-CoV and Middle East respiratory syndrome coronavirus have been included in recent clinical trials and exhibited encouraging results against SARS-CoV-2. Here, the authors provide a summary of therapeutic compounds that have shown antiviral effects against SARS-CoV-2 infections in cell lines, animal models, and patients.
Key Messages: With every passing day, knowledge about SARS-CoV-2 is increasing due to continued efforts of scientists working in this area globally. Approximately 15% of patients with COVID-19 are affected by severe illness and treatments are desperately needed. In this time of global pandemic, collective and co-ordinated efforts are needed to develop therapeutic agents against this disease.
INTRODUCTION
Severe cases of pneumonia of unknown aetiology from Wuhan, in the Hubei province of China, were reported to the World Health Organization’s (WHO) China Country Office on 31st December 2019. Later, public health officials reported that the causative agent of these pneumonia cases was a novel coronavirus, which was later renamed as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) on 11th February 2020.1 The WHO then named the disease caused by SARS-CoV-2 as the coronavirus disease 2019 (COVID-19). As of 14th July 2021, a total of 187,296,646 cases have been reported from 210 countries and territories, causing 4,046,470 deaths. Further, 3,327,841,570 vaccine dosages have been administered worldwide (at the time of writing).2
Most of the patients exhibited fever, dry cough, breathing difficulties (dyspnoea), headache, and pneumonia, bearing some resemblance with infections caused by SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). In severe cases of COVID-19, progressive respiratory failure due to alveolar damage, caused by acute respiratory distress syndrome (ARDS), led to the death of patients.3,4
Coronaviruses (CoVs), a group of enveloped, positive-sense, single-stranded RNA viruses, cause respiratory, hepatic, and enteric infections in several animal species including humans.5,6 Novel coronavirus SARS-CoV-2, of probable bat origin,7 belongs to the Coronaviridae family, which includes two subfamilies: Letovirinae and Orthocoronavirinae. The Orthocoronavirinae sub-family is further divided into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus.8 The past two decades have witnessed the emergence of three novel zoonotic Betacoronavirus: SARS-CoV, MERS-CoV,9 and the most recent SARS-CoV-2. Although most human CoVs do not cause severe disease, >10,000 cumulative cases were reported in the epidemics caused by SARS-CoV and MERS-CoV in the past two decades, with a mortality of 10% in the former and 37% in the latter.3
After the emergence of novel coronavirus SARS-CoV-2, several treatment and vaccination strategies have been envisaged and scientists across the globe are racing against time to develop successful antiviral drugs and vaccines. To date, seven vaccines have been launched globally; however, the surge of mutated strains of SARS-CoV-2 has deteriorated the situation further in many parts of the world, even 18 months into this pandemic. Vaccinating the whole population may take several years, hence the focus on the development of novel therapeutic interventions should be a global priority. Any new therapeutic intervention may require years to develop, so is of no use in the current scenario. Here, the authors have summarised the information available related to various treatment options, either approved or already in the developmental phase, for the infections caused by SARS-CoV or MERS-CoV. This review also focuses on the potential drugs that can be repurposed for the management of COVID-19.
THERAPEUTICS TARGETING VIRAL FACTORS AND PATHWAYS
The life cycle of SARS-CoV-2 consists of three main stages: entry into host cells through interaction with angiotensin-converting enzyme 2; replication of genome; and assembly of virions by exploiting the host cellular machinery. Potential therapeutic agents targeting these critical viral pathways exhibit antiviral effects. In this section, the authors have discussed the antiviral drugs or compounds that target viral factors and pathways required for their survival and spread inside the host (Figure 1).
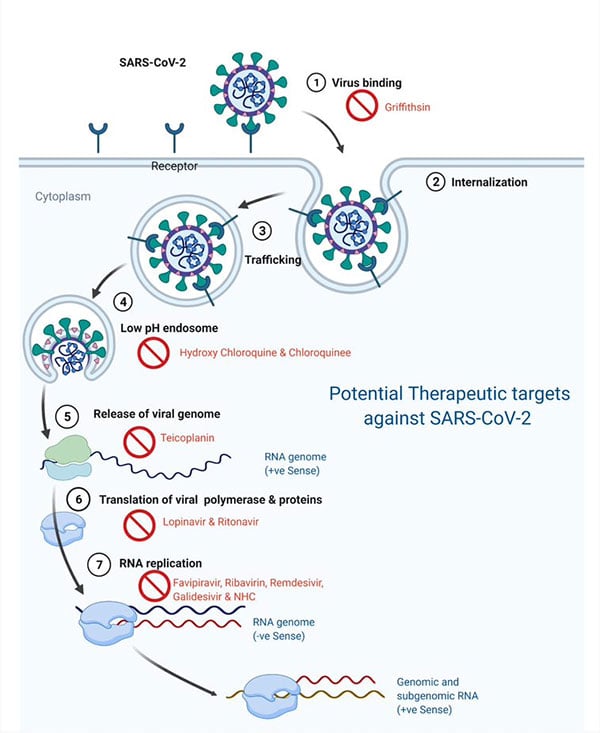
Figure 1: Directly acting therapeutic options against SARS-CoV-2.
NHC: an orally bioavailable cytosine analogue; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Image created using BioRender.
Nucleoside analogues
Nucleotides/nucleosides are the building blocks of nucleic acids including viral genomes. Nucleoside analogues are synthetic analogues of purine and pyrimidine bases, with altered sugar moiety or heterocyclic ring. These analogues are administered as prodrugs, which are converted into the active triphosphate inside the host cell by the action of host or viral kinases, and inhibit the viral replication by mis-incorporation of nucleotides in viral genomes during replication, causing chain termination that leads to the disruption of genome replication or subsequent transcription. Further nucleoside analogues may introduce mutations in the genome by substitution of natural nucleotides during chain elongation, leading to impairment of RNA synthesis, structure, and functions. Loss of viral fitness and viability by accumulation of mutations is called lethal mutagenesis.10,11 Drugs with the potential to target nucleosides interfere with viral genome synthesis and exhibit a broad range of activity against viruses including CoVs.
Favipiravir
Favipiravir (T-705), a guanosine analogue with approval for influenza treatment, inhibits the nucleic acid synthesis in RNA viruses such as influenza, Ebola, yellow fever, chikungunya, norovirus, and enterovirus.12 Its triphosphate form is incorporated in the viral RNA strand by the viral RNA-dependent RNA polymerase, resulting in chain termination and lethal mutagenesis.13,14 A recent study by Wang et al. reported the in vitro activity of favipiravir against SARS-CoV-2 in Vero E6 cells.15 Randomised clinical trials have been initiated in patients with COVID-19 to evaluate the safety and efficacy of a favipiravir and interferon-α combination (ChiCTR200002960016), and the efficacy and safety of a combination of favipiravir with two other drugs: baloxavir and barboxil (ChiCTR200002954417). Data obtained from these clinical trials will give a clear picture of the future usage of these drugs against SARS-CoV-2 infections. Meanwhile, on 19th June 2020, the Drugs Controller General of India (DCGI) granted approval to Glenmark Pharmaceuticals, Mumbai, India, to manufacture and market favipiravir for restricted emergency use for the treatment of patients with mild to moderate COVID-19, considering the unmet medical demands in India due to the ongoing pandemic.18
Ribavirin
Ribavirin, a guanosine analogue, exhibits antiviral activity by inhibiting the viral RNA synthesis and mRNA capping in a broad range of viruses, including CoVs. The monophosphate form of ribavirin interacts with nucleotide biosynthesis enzyme inosine monophosphate dehydrogenase, resulting in inhibition of guanosine production, which impairs the viral RNA synthesis.19 Moreover, its triphosphate form gets incorporated in the viral RNA by the viral polymerase, causing lethal mutagenesis.20 It has been approved for the treatment of hepatitis C virus and respiratory syncytial virus.21,22 However, when used in patients with SARS and MERS, it exhibited no obvious survival benefits. In a recent study, ribavirin exhibited antiviral effects against SARS-CoV-2 in Vero E6 cells.15 Pharmacokinetics and bioavailability data of this drug are available, and findings from ongoing clinical studies in patients with COVID-19 may highlight its antiviral efficacy against SARS-CoV-2 infections. In one completed clinical trial assessing the efficacy of triple combination of ribavirin, interferon β-1b, and lopinavir–ritonavir, Hung et al.23 reported that the ribavirin triple antiviral combination was superior and safer compared to lopinavir–ritonavir alone in treatment of patients with COVID-19.
Remdesivir
Remdesivir (GS-5734), one of the most promising drugs against SARS-CoV-2, is an adenosine analogue and a phosphoramidate prodrug with broad-spectrum activity against filoviruses, pneumoviruses, paramyxoviruses, and CoVs such as SARS-CoV and MERS-CoV.24 The triphosphate active form of remdesivir mimics adenosine triphosphate and acts as a substrate for viral polymerase. It is incorporated into the growing strand of viral RNA and causes the premature chain termination of viral RNA.25.26 Remdesivir is currently in clinical trial stage to evaluate its efficacy against Ebola.27 Furthermore, it reduced the viral load in the lungs of a murine model of SARS and MERS upon prophylactic and early therapeutic administration.28 Holshue et al.29 recently reported that a patient with COVID-19 recovered in the USA after intravenous treatment of remdesivir in January 2020. In a recent randomised, Phase III trial of remdesivir (Veklury®, Gilead Sciences, Foster City, California, USA), wherein 53 severely ill patients with COVID-19 received remdesivir on compassionate use basis, clinical improvement in 36 patients was observed; however, this study is limited by the lack of viral load data.30 In May 2020, Veklury received emergency use authorisation (EUA) from the U.S. Food and Drug Administration (FDA) for the treatment of hospitalised patients with severe COVID-19, after successful Phase III clinical studies.30 Additionally, many Phase III clinical trials have been initiated to evaluate intravenous remdesivir in patients with COVID-19 (NCT04252664,31 NCT04292730,32 NCT04292899,33 NCT04280705,34 and NCT0425765635), and the results are awaited.
Galidesivir
Galidesivir (BCX4430), an adenosine analogue, inhibits RNA polymerase activity in various RNA viruses including SARS-CoV, MERS-CoV, filoviruses, Ebola, and Marburg viruses.36 It is currently undergoing clinical studies for the evaluation of its safety and efficacy against yellow fever.37 In preclinical studies, it exerted antiviral activities in SARS and MERS, hence its efficacy against SARS-CoV-2 should be investigated. Recently, the National Institute of Allergy and Infectious Diseases (NIAID) initiated a randomised, double-blind clinical trial to investigate the antiviral effects of galidesivir in yellow fever and/or patients with COVID-19 and results are awaited (NCT0389142038).
β-D-N4-hydroxycytidine
NHC (β-D-N4-hydroxycytidine/EIDD-1931), an orally bioavailable cytosine analogue, has shown broad-spectrum antiviral effects against many RNA viruses, including Ebola, influenza, CoV, and Venezuelan equine encephalitis virus.39 Its antiviral effects are mainly attributed to the mutagenesis of viral RNA.40,41 In a recent study, Sheahan et al.39 reported that NHC/EIDD-1931 exhibited antiviral activity against MERS-CoV, SARS-CoV, SARS-CoV-2, and other Group 2b or 2c bat-CoVs, as well as the remdesivir-resistant CoV. Furthermore, prophylactic and therapeutic administration of EIDD-2801, an orally bioavailable NHC prodrug (β-D-N4-hydroxycytidine-5’-isopropyl ester), reduced the viral load and improved lung pathology in mice infected with SARS-CoV or MERS-CoV. This study could not demonstrate in vivo efficacy of NHC/EIDD-2801 against SARS-CoV-2 due to the lack of a suitable animal model. Collectively, broad-spectrum antiviral effects and oral bioavailability of NHC/EIDD-2801 highlight its therapeutic potential as an antiviral drug against SARS-CoV-2 infections.39 Currently, the efficacy of EIDD-2801 (molnupiravir) in patients with COVID-19 is being investigated in two clinical trials (NCT04405739,42 NCT0440557043).
Protease Inhibitors
The proteases expressed by viruses are involved in proteolytic cleavage of polyprotein precursors, a crucial step in the formation of fully functional viral proteins and processing of proteins essential for the assembly of viral particles. Thus, targeting the viral proteases by protease inhibitors interferes with the binding of substrates to binding sites of proteases and results in immature virus particles.44
Lopinavir and ritonavir
Lopinavir (LPV) is an approved HIV protease inhibitor, which is administered in combination with ritonavir (RTV), a cytochrome P450 3A4 (CYP3A4) inhibitor that enhances the efficacy of LPV. This combination has exhibited antiviral activity against SARS and MERS in addition to HIV-1.45 There are two proteases in SARS-CoV: a 3C-like proteinase and a papain-like cysteine proteinase.46 The antiviral effects of LPV/RTV against SARS-CoV-2 are believed to be mediated partially through the inhibition of the 3C-like proteinase.22,47
Recently, a controlled, randomised, open-label clinical trial was carried out to evaluate the efficacy of LPV/RTV in patients with the SARS-CoV-2 infection (n=199), which yielded no significant clinical benefit in those patients.48 However, another controlled clinical trial was initiated in China to evaluate the efficacy of a combination of LPV/RTV and IFNα-2b in patients with COVID-19 (ChiCTR200002930849) and outcomes are awaited. Despite the poor treatment outcomes in patients with COVID-19, the combination of LPV/RTV is still being prescribed to hospitalised patients in many countries and their efficacy is under investigation in several clinical trials across the world.
Griffithsin
Griffithsin is a red-alga-derived antiviral protein that attaches to the oligosaccharide moieties of viral glycoproteins such as glycoprotein 120 of HIV and SARS-CoV spike glycoprotein ‘S’, a major immunogenic protein of human CoVs, which plays an essential role in virus and host cell receptor interaction, and hence, offers a potential drug target.22 It inhibits a broad spectrum of viruses including SARS-CoV, human CoV NL63, and human CoV 229E in vitro as well as in mice infected with SARS-CoV.50,51 Further, griffithsin has been evaluated in a Phase I clinical trial as a gel or an enema for the prevention of HIV; nonetheless, safety and potency against COVID-19 should be evaluated.
Chloroquine and hydroxychloroquine
Chloroquine (CQ) and hydroxychloroquine (HCQ), age-old antimalarial drugs from the group of amino-quinolones, have shown activity against SARS-CoV-2 in virus-infected Vero E6 cells.15,52
CQ causes alkalinisation of the phagolysosomes by increasing endosomal pH, which blocks virus–cell fusion.53 Other mechanisms may include the inhibition of the glycosylation of viral proteins and cellular receptors of SARS-CoV. Few studies have reported the reduction of lung damage in patients with COVID-19 by chloroquine treatment, which may be attributed to modulation of cytokine release, an added benefit besides its antiviral properties.52
HCQ possesses a hydroxyl group in the side chain, which differentiates it from CQ.54 Like CQ, HCQ also increases endosomal pH and exhibits antiviral effects and modulates immune cells; however, HCQ is less toxic than CQ.55 The mechanism of action of HCQ is similar to that of CQ and it demonstrated a higher in vitro efficacy compared to CQ. The comparison of the half-maximal effective concentration values for HCQ (6.25 µM at 24 hours; 5.85 µM at 48 hours) and CQ (100 µM at 24 hours; 18.01 µM at 48 hours) revealed that HCQ exhibited a higher in vitro antiviral effect compared to CQ.53
CQ and HCQ are the most hyped drugs among all the potential therapeutics against COVID-19. The whole world seems divided on the role of these drugs in treatment of COVID-19. On the one hand, these drugs have been praised as the magic drugs and biggest game-changers in history of medicine by the then-president of the USA, Donald Trump. On the other hand, these drugs have been criticised as useless and dangerous in COVID-19 management.56
Two studies, each from France and China, were among the earliest to claim the therapeutic benefits of HCQ in COVID-19.57,58 However, the French study by Gautret et al.57 that used a combination HCQ and antibiotic azithromycin57 received criticism from the scientific community for drawing bold conclusions despite the lack of placebo arm, small sample size, and other limitations. Later, the International Society of Antimicrobial Chemotherapy (ISAC), which published this study in its journal, issued a statement that findings of the French study did not meet the society’s expected standards.59 On 28th March 2020, the U.S. FDA issued an EUA for the use of CQ and HCQ in the treatment of COVID-19.56
More than 100 clinical trials have been initiated in different parts of world to investigate the efficacy of CQ and HCQ in the treatment of COVID-19. The SOLIDARITY trial, co-ordinated by the WHO; DISCOVERY trial (SOLIDARITY in France); PRINCIPLE trial, conducted by the University of Oxford, UK; RECOVERY trial, also by the University of Oxford; and ORCHID trial, by the National Heart, Lung, and Blood Institute (NHLBI) in the USA, are the major clinical trials investigating the clinical benefits of HCQ in patients with COVID-19.60 Major controversy erupted when analysis of a multicentre registry of 96,032 patients by Mehra et al.61 reported enhanced ventricular arrhythmias and reduced survival of hospitalised patients with COVID-19 receiving CQ and HCQ, with or without a macrolide. Consequently, the DISCOVERY and SOLIDARITY trials were suspended immediately. However, this article was retracted within two weeks of publication and the WHO resumed the SOLIDARITY trial. Further, the RECOVERY investigators concluded that HCQ failed to exhibit any beneficial effect in patients with COVID-19 and discontinued the HCQ arm of the trial immediately. In mid-June 2020, the U.S. FDA determined that HCQ and CQ were ineffective in treatment of COVID-19 and revoked the EUA, leading to the discontinuation of the HCQ arm from the SOLIDARITY and ORCHID trials.60
Teicoplanin
Teicoplanin, a glycopeptide antibiotic currently used to treat staphylococcal infections, has shown broad-range antiviral activity against influenza virus, flavivirus, hepatitis C virus, Ebola virus, and human CoVs such as SARS-CoV and MERS-CoV.62 Teicoplanin inhibits the cathepsin-L-mediated cleavage of the viral spike protein in the late endosomes, thus blocking the release of viral RNA and subsequent virus replication cycle of CoVs.62 A recent study by the same group reported that teicoplanin exerts in vitro antiviral effect against SARS-CoV-2.63 Further investigation of this drug should be carried out to evaluate its efficacy in animal models.
HOST-DIRECTED THERAPEUTICS AGAINST SARS-CoV-2
SARS-CoV, MERS-CoV, and SARS-CoV-2 induce unusual and extreme host immune responses, causing severe lung pathology. Similar to SARS and MERS, some patients with COVID-19 develop ARDS and in many of the moribund patients cytokine storm is observed, which is characterised by the release of IL-2, IL-7, IL-6, granulocyte-macrophage colony-stimulating factor, IFN-γ, IL-12, IFN-γ-induced protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and TNF-α.3,64,65 This aberrant immune response causes fibrosis and long-term lung damage, leading to reduced pulmonary function and a low quality of life in the patients who survive the intensive care.66,67
Development of therapeutics specific to SARS-CoV-2 may take years; in the meantime, existing host-directed therapeutics with proven safety profiles may be repurposed for use in treatment as adjuncts to combinational therapies with cyclosporine, remdesivir, lopinavir–ritonavir, IFN-β-1b, antiviral peptides, and monoclonal antibodies targeting SARS-CoV-2. These agents can help in boosting the immune system, reducing lung immunopathology, and preventing the COVID-19-associated ARDS.
Tocilizumab
Tocilizumab, a monoclonal antibody against the IL-6 receptor (licensed for both rheumatoid arthritis and cytokine release syndrome) with a proven safety profile, can be used as an adjunct to antiviral treatments. IL-6, a proinflammatory cytokine secreted by macrophages, fibroblasts, and T- and B-cells, plays an important role in several immunological processes such as Ig secretion, T-cell activation, and initiation of hepatic acute-phase protein synthesis. Tocilizumab binds to both soluble and membrane-bound IL-6 receptors, and thus interferes with IL-6-mediated signalling. A recent retrospective study of 21 patients with COVID-19 using tocilizumab reported improved lung condition on CT scans, oxygen concentration, normalised lymphocyte counts, and C-reactive protein levels in most of the patients.68 Furthermore, a randomised clinical trial of tocilizumab has been initiated in China in patients with COVID-19 pneumonia and elevated IL-6 levels (ChiCTR200002976569). A retrospective, observational cohort study reported reduced mortality in patients with COVID-19 admitted to the intensive care unit after treatment with tocilizumab.15 Another retrospective cohort study from India reported survival benefits of tocilizumab in severely ill patients with COVID-19 with persistent hypoxia.70 Recently, the Italian Medicines Agency (AIFA) has also approved a Phase II clinical trial of tocilizumab in an estimated 330 patients with COVID-19 pneumonia.68
Anakinra
Anakinra is a IL-1 receptor antagonist that blocks the activity of cytokine IL-1, which is critical to the cytokine storm; recent studies have reported that SARS-CoV-2 infection leads to pyroptosis with the increase in the levels of IL-1β.71,72 A Phase III randomised clinical trial of anakinra in patients with severe sepsis and macrophage activation syndrome demonstrated significantly improved incidences of patient survival.73 Currently, data related to the potential use of anakinra in SARS-CoV-2 infections are limited; nonetheless, its evaluation in patients with COVID-19 and ARDS and cytokine storm may be worthwhile as it may provide an alternative therapeutic option in the management of patients with COVID-19. In a recent cohort study, anakinra significantly reduced the mortality and ventilation in patients with COVID-19 with advanced complications.74
Adalimumab
Adalimumab is a monoclonal antibody against human TNF-α, marketed for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, and ulcerative colitis. The spike protein of SARS-CoV induces shedding of the angiotensin-converting enzyme 2 ectodomain in a TNF-α-converting enzyme-dependent manner that is crucial for viral entry into the cell.75 TNF-α is placed at the centre of this process; hence, it has been hypothesised that the use of TNF blockers may have an effective role in the reduction of SARS-CoV-2 infection and subsequent organ damage.76 Lately, a study to evaluate the efficacy of adalimumab in patients with COVID-19 has been initiated in China (ChiCTR200003008977).68
Convalescent plasma
Convalescent plasma is plasma obtained from patients who have recovered from infection, and it contains neutralising antibodies against that particular microbe. It has been used as a last resort in patients with SARS to improve survival benefit. Furthermore, several studies have reported lower mortality and shorter hospital stays in admitted patients receiving convalescent plasma treatment compared to control groups.78,79 In 2014, the WHO recommended use of convalescent plasma as empirical treatment in patients infected with the Ebola virus.80 Antibodies present in the convalescent plasma might reduce the viraemia in patients, which may be the reason for the efficacy of convalescent plasma therapy.
In most viral diseases, viraemia peaks within the first week of infection, followed by generation of primary immune response by Day 10–14 in most patients, leading to viral clearance. Therefore, convalescent plasma therapy should be more effective if it is started at the early stage of the disease;78 however, other treatments including steroids and antiviral drugs might affect the antibody level in the convalescent plasma.81
In a preliminary, uncontrolled case study, Shen et al.82 administered convalescent plasma to five patients who were severely ill with COVID-19 with ARDS, and reported an improvement in their clinical status. Briefly, body temperature was normalised in 4/5 patients within 3 days of plasma transfusion. Viral loads decreased significantly and ARDS was resolved within 12 days post-transfusion. Due to the limited sample size of the study, definitive statements about the efficacy of convalescent plasma therapy cannot be made and these findings should be evaluated in clinical trials.82 This evidence suggests that convalescent plasma therapy may have a potential role in the management of patients critically ill with COVID-19. Therefore, it seems plausible to evaluate the safety and efficacy in future clinical trials.
Corticosteroids
Corticosteroids, a class of anti-inflammatory drugs, have been proven to be useful in severe cases of COVID-19 associated with aberrant immune activation leading to cytokine storm and subsequent ARDS. A recent trial (NCT0432740183) using dexamethasone in patients with COVID-19 with severe ARDS reported significant survival benefits compared to the placebo group.84 The RECOVERY trial data suggested that dexamethasone lowered the 28-day mortality in patients with COVID-19 receiving oxygen support or mechanical ventilation; however, it is not recommended for use in patients with COVID-19 with mild or moderate symptoms without the need for oxygen support.85 Due to the dynamic nature of the immune activation, corticosteroids should be given at the right time and right stage of disease progression to achieve maximum benefits.
CONCLUSION
As SARS-CoV-2 continues to spread and the death toll rises exponentially across the globe, rapid development of therapeutic interventions against SARS-CoV-2 infections to minimise COVID-19-related deaths poses a major challenge. In the present scenario, repurposing existing antiviral drugs with known safety profiles and, in few cases, their efficacy against other human CoVs, seems to be the most appropriate short-term strategy to tackle COVID-19.
Currently, several potent antivirals such as lopinavir–ritonavir, remdesivir, CQ/HCQ, etc., with or without other supporting medications, are being administered to patients with COVID-19 in various clinical trials; however, sufficient data are still lacking. It is pertinent to mention that, besides identifying suitable therapeutic options, intense and concerted efforts are also being directed towards development of appropriate vaccines. In this direction for controlling this ongoing pandemic, several vaccine candidates, including spike protein, attenuated live viral particles, and Mycobacterium bovis bacillus Calmette-Guérin-based interventions are either launched in the market or being tested in clinical trials. However, since the present article focuses only on the therapeutic options against COVID-19, this aspect has not been discussed.
The whole world is united in its efforts to prevent the spread of SARS-CoV-2 with a hope that this pandemic meets the fate of the previous two pandemics of this century; SARS and MERS have subsided on their own. However, only the future can tell the endpoint of this pandemic. Moreover, with the rapid rise of mutant variants across the globe, one thing is clear: there is a pressing need to develop wide-spectrum antiviral therapeutics against SARS-CoV-2 to reduce the mortality it is causing. Therefore, intense research and development efforts and development of multi-pronged therapeutic approaches for combating this pandemic will constitute a top priority research area in virology.





