Abstract
An estimated 11 million workers in the USA are potentially exposed to agents that can become a cause of allergic diseases such as occupational asthma and allergic contact dermatitis, which can adversely affect health and well-being. Hundreds of chemicals (e.g. metals, epoxy and acrylic resins, rubber additives, and chemical intermediates) and proteins (e.g. natural rubber latex, plant proteins, mould, animal dander) present in virtually every industry have been identified as causes of allergic disease. In general, allergens can be classified as low molecular weight (chemical) allergens and high molecular weight (protein) allergens. These agents are capable of inducing immunological responses that are both immunoglobulin E and non-immunoglobulin E-mediated. Interestingly, the same chemical can induce diverse immune responses in different individuals. As new hazards continue to emerge, it is critical to understand the immunological mechanisms of occupational allergic disease. Specific understanding of these mechanisms has direct implications in hazard identification, hazard communication, and risk assessment. Such efforts will ultimately assist in the development of risk management strategies capable of controlling workplace exposures to allergens to prevent the induction of sensitisation in naïve individuals and inhibit elicitation of allergic responses. The purpose of this short review is to give a brief synopsis of the incidence, agents, mechanisms, and research needs related to occupational allergy.
INTRODUCTION: INCIDENCE OF OCCUPATIONAL ALLERGY
Occupational immune diseases are among the most common illnesses that affect workers. An estimated 11 million workers in the USA, across every industrial sector, are potentially exposed to agents that can produce allergic diseases including: asthma, allergic contact dermatitis (ACD), urticaria, allergic rhinitis, eczema, and folliculitis.1 Significantly, occupational exposures are responsible for approximately 9–25% of all adult onset asthma cases,2,3 while ACD represents 20% of all work- related cutaneous disorders.4 These diseases can adversely affect an individual’s health and capacity to perform at work, resulting in significant economic losses.5,6 Similar findings have been reported in Europe and other developed nations where occupational allergens are a recognised health hazard.7
Occupational asthma and ACD have been reported to show increased incidence in healthcare workers;8,9 hairdressers and cosmetologists;10,11 individuals working in manufacturing and automotive industries;12,13 cleaning and janitorial staff;14,15 food processing and packaging workers;16,17 animal handlers;18 and individuals working with metals,19 compared to individuals in other occupational sectors. Over 250 causative agents of occupational asthma have been reported20 and approximately 400 allergens are available for patch testing in humans,4 demonstrating the breadth of potential allergens found in the workplace.
TYPES OF DISEASE
For the purpose of this manuscript, only immunological allergic diseases will be reviewed. The severity of allergic disease can be influenced by several factors including the route of exposure, the source of exposure, the environment, and genetics. Allergic diseases are characterised by a latency period between exposures (sensitisation) and symptoms (elicitation) and may involve immunoglobulin E (IgE) and non-IgE-mediated responses. In the context of hypersensitivity or allergic reactions there are four basic hypersensitivity reactions as originally classified by Gell and Coombs in 1963.21 The distinct responses were characterised based on the primary effector molecules and immune cells involved in each reaction. Type I and Type IV (referred to as IgE and non-IgE-mediated, respectively) are the most common hypersensitivity reactions in the occupational setting. While in recent years these classification schemes have been further subcategorised, the importance of the role of the innate immune system in allergy is increasingly being recognised. These concepts are beyond the scope of this review.22,23
Immunoglobulin E-Mediated
An IgE-mediated allergic reaction is mediated by IgE antibody and mast cells and is sometimes called immediate-type hypersensitivity (Type I). It involves the initiation of T helper 2 cytokines, such as interleukin (IL)-4 and IL-13, leading to IgE production by B cells. Once IgE is produced and secreted, it binds to mast cells and basophils. Upon activation, these cells degranulate and release soluble allergic mediators, such as histamine and leukotrienes, which act on smooth muscles, sensory nerves, mucous glands, arteries, and eosinophils.24 Common clinical outcomes of an IgE-mediated reaction are increased vascular permeability, smooth muscle cell contraction, and vasodilation. IgE-mediated reactions manifest within minutes to hours of exposure. Depending on the site(s) and frequency of allergen exposure, these reactions may occur in one or more organs resulting in diseases such asthma, allergic rhinitis, urticaria, and anaphylaxis.
Non-Immunoglobulin E-Mediated
A non-IgE-mediated or delayed type hypersensitivity response (Type IV) is T cell- mediated and characterised by excessive inflammation. The most distinctive feature of a non-IgE-mediated hypersensitivity response is the delay observed between allergen exposure and immune response. Following sensitisation, subsequent exposures result in elicitation of the non-IgE-mediated response, characterised by the secretion of proinflammatory cytokines (granulocyte-macrophage colony-stimulating factor, interferon- IL-3, IL-12, and tumour necrosis factor-) that activate and recruit macrophages and other immune cells. Due to the time it takes for these cytokines to attract and activate macrophages at sites of exposure, the effector phase typically occurs 24 hours following exposure and it generally peaks at 48–72 hours after exposure.24 ACD is an example of a non-IgE-mediated hypersensitivity reaction.
OCCUPATIONAL ALLERGENS
Occupational allergens encompass a wide variety of substances. This includes both proteins and chemicals, high and low molecular weight (HMW/LMW) compounds, and natural and synthetic products (Table 1). Some of the most common allergens are wheat and enzymes (bakeries); latex, antimicrobials, and biocides (healthcare workers); isocyanates and anhydrides (manufacturing); nickel and cobalt (metal workers); and persulphates (hairdressers). Typically, occupational allergens are classified as either HMW >5 kDa, or LMW <5 kDa, and their size is thought to play a significant role in their allergenicity and mechanism of action. Protein allergens are usually HMW, while chemical allergens are LMW. HMW agents act as complete antigens and are innately immunogenic, whereas LMW chemicals must first react with autologous or heterologous proteins to form a hapten-complex before they can act as a functioning allergen. IgE responses are most commonly seen following HMW antigen exposure but can also be seen following LMW exposures. Metal ions such as nickel, cobalt, and chromium are among some of the most common triggers of ACD.25 However, much less is known about the immunological responses to metals.26 In addition to frequent exposure, other factors, such as predisposing skin injuries, atopy, and genetics, may influence an individual’s susceptibility to developing allergies.
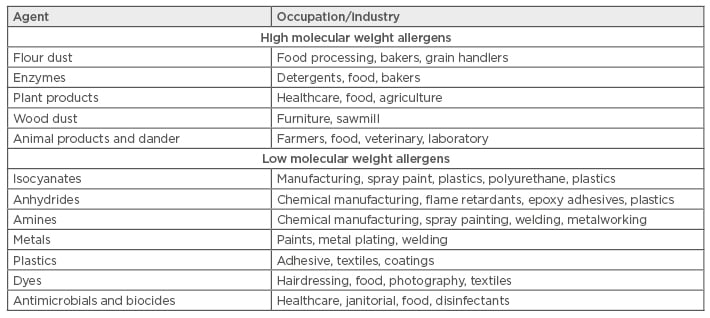
Table 1: Common occupational allergens.
Low Molecular Weight Occupational Allergens
LMW chemical allergens are diverse in structure, reactivity, and application; however, there are several common attributes that are associated with immunogenicity including: haptenation potential (protein reactivity), ability to access the epithelium, and irritancy potential.27,28 Thousands of chemicals have been identified as causative agents of skin sensitisation resulting in ACD, while substantially fewer chemical allergens (<100) have been identified as causative agents of asthma.29 For the majority of LMW sensitisers, the immunologic response has no proven mechanisms and often can result in non-IgE and IgE-mediated responses.27
One of the most common occupationally-relevant chemical allergens, toluene diisocyanate (TDI), is a highly reactive chemical utilised in the automobile industry and in the manufacture of polyurethane foams, paints, elastomers, and coatings. TDI is a potent allergen and exposure can lead to a variety of diseases, including asthma, rhinitis, and ACD.20,30 The incidence of asthma related to occupational TDI exposure has been estimated at ≤5.5% for the total workforce.13 Based on the majority of the available epidemiological data, persulphate salts are reported as another common occupational allergen and may cause ACD, urticaria, rhinitis, and asthma.10,11 Persulphate salts (ammonium, potassium, and sodium) are inorganic salts used as oxidising agents in hair bleaches and hair-colouring preparations at concentrations of ≤60%.31 TDI and persulphate salts are generally classified as IgE-mediated sensitisers but may also induce a non-IgE-mediated response.32,33 However, while animal studies support an IgE-mediated mechanism, TDI asthmatics often have no measurable TDI-specific IgE. Similar findings have been reported for persulphate.34,35 The complete immunological mechanisms of sensitisation for these chemicals and other LMW sensitisers are not fully understood.
Numerous LMW chemical allergens are used in the healthcare profession. These include biocides (formaldehyde, glutaraldehyde, and orthophthaldehyde) commonly used to sterilise medical devices that are sensitive to normal heat or steam sterilisation processes and as disinfectants for surfaces (quaternary ammonia compounds).36 Aldehydes and quaternary ammonia compounds have been identified as some of the most common non-IgE-mediated allergens.25 In addition, medical gloves containing certain rubber accelerators (thiuram mix and carba mix), and antibacterial hand sanitisers and soaps (chloroxylenol and cocamide diethanolamine), have also been identified as common sources of allergens.36 The above examples represent some of the most common occupational LMW allergens; however, many other occupationally relevant LMW allergens exist.
High Molecular Weight Allergens
Since the majority of allergies induced by HMW allergens are IgE-mediated, detection and quantification of specific IgE that recognises the responsible protein is used for confirmation of allergy. This can be evidenced through positive skin prick tests or immunoassays.37 Several challenges exist in the diagnosis and identification of HMW allergy. The HMW allergens in some compounds, such as wheat and latex, have been better characterised than others. Additionally, while most recombinant proteins are available for testing, multiple proteins may be responsible and individuals may have different sensitivities to different proteins which may present a challenge for the identification of the suspect agent.37 In addition, LMW chemicals may be a component of the crude allergen (introduced via processing or manufacturing) and may also result in non-IgE-mediated responses.
It is estimated that 6–17% of healthcare workers suffer from latex allergy, with rubber gloves being the most common cause.38 Latex allergy can manifest as urticaria, rhinitis, conjunctivitis, asthma, anaphylaxis, and ACD. Latex is extracted from the Hevea brasiliensis tree (rubber tree) and contains an array of cellular proteins, lipids, and amino acids. The responsible allergens in latex have not been fully characterised but a list of 15 allergens (Hev b 1–Hev b 15) has been established with Hev b 5, Hev b 6.01, and Hev b 6.02 identified as the most common occupational latex allergens.39 Chemicals such as thiurams, stabilisers, and antioxidants (thiocarbamates, diphenylamine, dihydroquinoline, and phenylenediamine), which may be added to the latex during the manufacturing of rubber, have been recognised to induce ACD.40
Flour is another very common HMW occupational allergen and epidemiological reports have revealed that asthma, rhinitis, and ACD are the major health effects due to exposure.41 Flour is a complex organic dust containing cereals which have been processed by milling. Flour dust usually contains various components which play an important role in dough improvement, such as a variety of enzymes (-amylase, cellulose, hemicellulose, malt enzymes), additives (baker’s yeast, egg powder, milk powder, sugar), flavourings, spices, and chemical ingredients (preservatives, antioxidants, bleaching agents). Wheat is the main flour used in the baking industry and has been found to contain at least 40 allergens which represent about 10–15% of the dry weight of the grain.42 Baker’s asthma is one of the most frequently occurring forms of occupational asthma and most studies indicate that wheat and rye flour proteins are allergens for 60–70% of bakers with workplace-related respiratory problems.43 The enzyme -amylase (added to improve baking characteristics), thioredoxin, plain lipid transfer proteins, and serine proteinase inhibitors are among the main factors associated with baker’s asthma and studies have found that the highest frequency of specific IgE measurements were identified for -amylase inhibitors Tri a 28 and Tri a 29.01.41 Chemical components in flour such as preservative and bleaching agents have also been shown to cause ACD in bakers.44
Exposure to laboratory animals has been shown to result in occupational allergy and is commonly observed among technicians, animal caretakers, physicians, and scientists who work in pharmaceutical industries, university laboratories, and animal breeding facilities.45 Rodents such as mice and rats, that are frequently used in animal research, are the most common causes of occupational allergy to laboratory animals. Mouse sensitisation is increasing in laboratory animal technicians and researchers due to the dramatic increase in the use of mice in experimental models. It is estimated that between 5% and 8% of this population is affected with some estimates suggesting an increase of ≤23% over a 2-year period in the USA. Urine is the main source of the allergenic protein in both mice and rats but allergens can also be found in dander, hair, saliva, and serum.46 As with most mammals, the major inhaled allergens in mice and rats are lipocalins (Mus m 1 and Rat n 1, respectively). These allergens share 64% homology between their amino acid structures. Mouse urinary protein has shown IgE cross-reactivity with rat urinary protein and Equ c 1 (a major horse allergen).47
Metals
Metals are considered to be one of the most common occupational allergens and it is estimated that 10–15% of the population have allergies to at least one species of metal.48 Occupational exposure to metals can result in varying levels of morbidity and mortality due to the induction of a wide range of allergic diseases including ACD, occupational asthma, and anaphylaxis. Surprisingly, little is known about the immunologic mechanisms driving the reaction behind metal allergy.26,48 Metals are thought to interact directly with the surface of human lymphocytes to stimulate the adaptive immune response, however the exact mechanism is not fully understood.49 Recent research also supports a role for the involvement of the innate immune system (specifically toll-like receptors) in the allergic responses to metals.50 Numerous metals including gold, chromium, cobalt, platinum, nickel, palladium, and mercury are known to induce allergic responses resulting in ACD and asthma.19 Following patch testing of 4,454 patients (not all due to occupational exposure), nickel sulphate (19.0%), cobalt chloride (8.4%), and potassium dichromate (4.8%) were among the most common allergens, with nickel being identified as the most frequent positive allergen.19 Sources of occupational allergen exposure include releases from dental tools and alloys,51 scissor and nail instruments used by cosmetologists and nail technicians,52 coin handling operations,53 and metal processing.54
Challenges and Research Needs
Basic research
As new potential allergens are identified, it is critical that we fully understand the immunological mechanisms of occupational allergic disease. Research is needed to fill gaps in basic knowledge about the hazards of these agents. Areas of interest include: i) elucidating the mechanisms of allergic disease, ii) identifying exposure assessment biomarkers, iii) describing the role of genetics and the environment in allergic disease, iv) characterisation of complex exposures leading to allergic diseases, and v) developing predictive testing for the identification of occupational allergens.
The classification of allergens, especially LMW allergens, has often proven to be difficult since studies have identified that exposure to certain chemicals can result in multiple hypersensitivity pathways (i.e. both ACD and asthma). A more complete and thorough understanding of the immune-mediated mechanisms is needed before we will be capable of identifying, preventing, and treating allergic diseases. The need for the identification of potential exposure assessment biomarkers for sensitisation and exposure-sensitisation response relationships of occupational sensitisers is also imperative. Recently, many novel cellular subsets and molecules potentially involved in immunological allergic responses have emerged as potential candidates for biomarkers. The identification of the potential involvement of novel T helper subsets and non-coding RNA elements, such as microRNAs55 in allergic disease, illustrates the advancement of these research needs. Additional studies are necessary to determine the relative role of individual versus complex workplace exposures in the development of allergic disease. This is of concern because investigations of individual chemicals may not adequately reflect the mixed exposures that often occur in occupational settings. Understanding the role of genetics and the environment on the allergic response is also critical.56 An example of the importance of genetic factors in susceptibility to allergic disease is the influence of human leukocyte antigen genes on TDI asthma susceptibility. Several studies involving TDI-exposed workers demonstrated that specific human leukocyte antigen Class II genotypes were over-represented in asthmatic workers compared to asymptomatic workers.56 Characterising the role of exposure route is another substantial challenge. Historically, the focus has been on describing the toxicity associated with the inhalation of hazardous substances. Available evidence clearly demonstrates the role of the skin as an important organ in respiratory disease. Factors such as skin integrity have been shown to influence sensitisation and the development of the respiratory allergic response.57 However, additional research is needed to fully understand the role of the skin in respiratory allergic disease. Immunological assessment for occupational allergens is limited by the fact that standardised tests are not available for most workplace-relevant allergens. Predictive tests are critical for early identification of the hazard. It is understood that allergens may induce multiple types of allergic reactions. This is especially true for LMW allergens that can induce IgE and non-IgE-mediated responses. Due to the incomplete knowledge regarding mechanisms, predictive tests are lacking for these kind of exposures. Early detection of preclinical biomarkers of sensitisation may prevent development of occupational diseases through the implementation of the proper administrative and engineering controls.
Applied Research
Occupational allergy has significant social and economic implications for workers, their families, their employers, and government agencies. Sensitised workers must avoid exposure to the allergen both at work and outside the workplace in order to have the best chance of improvement or clearing of the allergic manifestations. This may be achieved by altering workplace tasks and duties, implementing engineering controls, or by providing workers with appropriate personal protective equipment. Most often, a sensitised worker would have to move to a completely different area or change to a different workplace or occupation to avoid further exposure to the offending allergen. However, numerous approaches that integrate risk assessment and risk management strategies have been developed to control workplace exposures to occupational allergens.58-61 In this context, it is imperative to establish an effective risk management strategy that is designed to prevent the induction of sensitisation in naïve individuals and inhibit elicitation of allergic responses in those that have become sensitised.2 Such a strategy should include both primary and secondary prevention methods. Primary prevention methods are interventions used to prevent worker sensitisation and may include the following:
- Modification of the allergen to inhibit exposure
- Application of control methods to prevent exposures
- Substitution with a less harmful agent
- Use of personal protective equipment62
Secondary prevention methods attempt to characterise workplace exposure, in addition to detecting and limiting the progression of allergic diseases. Examples of secondary preventive methods include medical monitoring58,62,63 and workplace exposure monitoring.2,58,61
Another important tool applied to characterise and aid in controlling workplace exposures to occupational hazards are occupational exposure limits (OELs). Despite their widespread use globally, few OELs are established on the basis of preventing sensitisation. The quantitative risk assessment approaches used to derive OELs have been developed primarily for non-immune-mediated effects, such as portal of entry effects, non-cancer systematic effects, or cancer. Application of these approaches to develop OELs for allergens has been inhibited because of data limitations and a lack of understanding of the biological processes that govern immune-mediated effects. The route of exposure, exposure intensity, and duration/ frequency of exposure have also been identified as factors complicating this process.2 Research addressing these challenges along with a better understanding of allergic disease has direct implications in hazard identification, informing appropriate risk assessment, and management decisions to facilitate interventions and prevention of occupational allergies.





