Meeting Summary
The symposium provided a flavour of key presentations and issues discussed at the 2017 Future of the Allergists and Allergen Specific Immunotherapy (FASIT) workshop held in Hamburg, Germany. Prof Wahn explained that the FASIT meeting brought together basic scientists, clinicians, and practitioners to brainstorm issues around allergy. Prof Jutel considered FASIT presentations exploring how precision medicine can be used to select optimal patients for allergen immunotherapy (AIT). He outlined the role of phenotypes and endotypes, reviewed some biomarkers that are currently under validation, and considered the role of adjuvants. Prof Kleine-Tebbe considered a number of promising Phase II studies that have failed to be translated into successful Phase III studies. Factors influencing results, he said, include high placebo effects, natural variability of the environment, patient heterogeneity, and the use of different endpoints for Phase II and III trials.
Prof Pfaar considered whether allergen exposure chambers (AEC) are at a stage to be used for Phase III (pivotal) trials in AIT. He provided an overview of the history and advantages behind these facilities and reported the regulatory view. Prof Pfaar reported a recently published Position Paper from the European Academy of Allergy and Clinical Immunology (EAACI) addressing the current status of allergen chambers and setting the ‘frame’ for further developments, such as clinical validation. The position paper included the views of all relevant stakeholders, including clinicians, chamber operators, and regulators. In the second part of his talk, Prof Pfaar reviewed the introduction of paediatric investigation plans (PIP), which are required for allergen products prior to receiving marketing authorisation and considered the methodological problems for fulfilling these regulatory demands. Finally, Prof Pfaar called for further consultation and collaboration between all parties involved in AIT regarding possible improvement of PIP.
Prof Kuna highlighted the European Union (EU) Directive 2001/83EC, which threatens both allergy diagnostics and the entire discipline of allergology. The directive states that allergens should be considered as drugs, and makes no distinction between allergens used for therapeutic procedures and those used for diagnostic purposes. The cost of obtaining and keeping marketing authorisations for test allergens is expensive. Already there are signs that allergen testing has been reduced in Europe. Prof Kuna concluded that it is essential for all stakeholders (authorities, allergists’ societies like the EAACI, the European Medicines Agency (EMA), European legislators, and allergen manufacturers) to come together to ensure the continued availability of in vivo allergen diagnostic tests in the EU.
Introduction
Professor Ulrich Wahn
Prof Wahn explained that Allergopharma (Reinbek, Germany) invites international leaders in the allergy field to come together to discuss important immunotherapy issues. The initiative led to the first FASIT workshop in 2006, with meetings now held regularly. Allergists, said Prof Wahn, provide holistic overviews of various chronic (allergic) diseases, summarised as atopic syndrome. They evaluate triggers and causes of allergy, provide evidence-based recommendations on environmental control, prescribe pharmacotherapy, give advice on prevention, and educate primary care physicians. AIT is a “game changer”, said Prof Wahn. It not only offers symptom relief, but modifies disease. FASIT, bringing together basic scientists and clinicians, provides a platform for research updates and opportunities for informal brainstorming around immunology and its clinical and regulatory requirements.
Europe as a continent, said Prof Wahn, illustrates how diverse the practice of immunotherapy can be. Data from the World Allergy Organization (WAO) in 2010 showed that in the UK just 1,700 specific immunotherapy treatments have been applied compared to 700,000 in Germany, 500,000 in France, 380,000 in Italy, and 350,000 in Spain.1 Even for countries strong in AIT there is variation in the way treatment is delivered. Subcutaneous immunotherapy (SCIT) dominates in Germany and Spain, while sublingual immunotherapy (SLIT) dominates in France and Italy. FASIT provides the forum for debating differences in practice. FASIT, Prof Wahn concluded, stands for progress, innovation, and open discussion.
New Promising Perspectives in Allergen Immunotherapy
Professor Marek Jutel
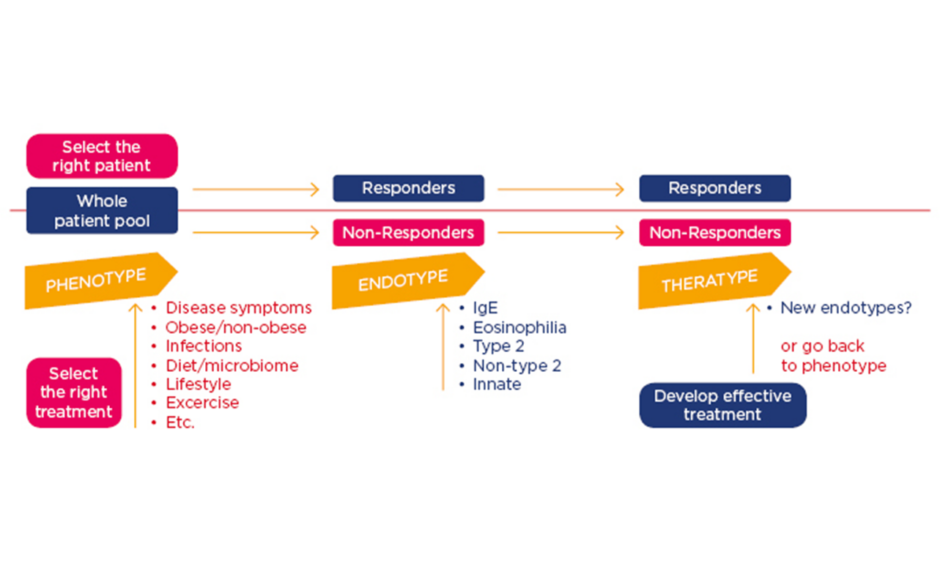
The concept of personalised medicine, Prof Jutel explained, offers promise to develop tools predicting who will respond to therapies, thereby creating the opportunity of targeted treatments. The ideal approach involves identifying the phenotype (defined as visible properties), exploring the endotype (pathogenic mechanisms), and optimising the treatment for the patients (theratype) (Figure 1). Precision medicine in AIT helps selection of the optimal patient and therapy to reduce trial-and-error prescribing and control the overall costs.
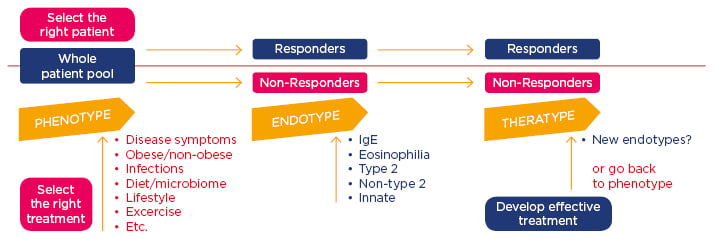
Figure 1: Personalised and precision approach for treatment of allergic patients.
Jutel M. FASIT “Asthma phenotyping and endotyping – precision medicine”.
Ig: immunoglobulin.
One study showed certain endotypes (namely high or low expression of monocyte chemotactic protein-1 [MCP-1], eotaxin, and IL-8) distinguish patients according to disease severity (whether mild, moderate, or severe) in eosinophilic asthma.2 Biomarkers, Prof Jutel said, can be used to link endotypes to clinical AIT phenotypes, leading to improved patient stratification.3
An EAACI task force, led by Mohamed Shamji, recently assessed a range of biomarkers for utility as surrogate/predictive markers of efficacy for clinical trial use and monitoring of individual patient responses.4 The task force, which reviewed seven domains of biomarkers (Figure 2), concluded that intracellular expression of fluorochrome-labelled diamine oxidase in basophils might be suitable as a biomarker for efficacy and tolerance after AIT. Furthermore, they concluded differential expression of DC2 (GATA-3, CD141, and RIPK4) and DCreg (C1QA and FcyRIIIA) cell markers following SLIT to likely be associated with efficacy.
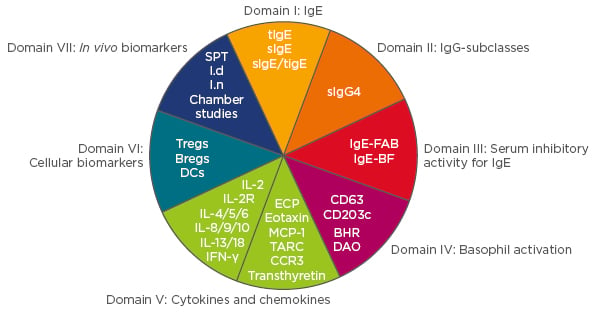
Figure 2: Domains reviewed by the EAACI Biomarkers task force.3
Each biomarker was assessed on the basis of its utility as a surrogate/predictive marker of efficacy in clinical trials and for monitoring the response of patients in clinical practice.
Ig: immunoglobulin; tlgE: total IgE; sIgE: specific IgE; sIgE/tIgE: specific IgE/total IgE ratio; Tregs: T regulatory cells; Bregs: B regulatory cells; DCs: dendritic cells; SPT: skin prick test; Id: intradermal; In: intranasal; IL: interleukin; IFN-γ: interferon gamma.
Shamji M. FASIT 2017 “Biomarkers in Allergen Immunotherapy”.
Adapted from Shamji et al.4
Other studies suggested additional biomarkers for AIT efficacy. In one study, Shamji et al.5 showed that functional assays of inhibitory immunoglobulin (Ig)G4 and IgE-blocking factors were more useful surrogates of clinical response than IgG4. In a second study, Shamji et al.6 found expression of fluorochrome-labelled diamine oxidase (DAO) in whole blood basophils was significantly higher among patients who achieved successful AIT (both SCIT and SLIT) for seasonal allergies. Gueguen et al.7 showed that a combination of five markers (C1Q, CD141, FcγRIIIA, GATA3, and RIPK4) correlated with AIT SLIT clinical efficacy.
In real life, said Prof Jutel, factors such as obesity, diet, the microbiome, lifestyle, and exercise all influence efficacy. One factor is histamine, the effect of which on immune regulatory cells has been known for several decades.8-10 In a recent study, Barcik et al.11 found levels of the histamine secreting bacteria M. morganii in the human gut microbiome correlated with asthma disease severity (mild, moderate, or severe). On the basis of such observations, Barcik et al.11 speculated that increased levels of bacterial derived histamine in asthma patients contribute to histamine-related pathologies.
There is also evidence that diet influences microbiome composition. In controlled feeding studies, Wu et al.12 showed that microbiome composition changed detectably within 24 hours of initiating a high-fat/low-fibre or low-fat/high-fibre diet, but that enterotype identity remained stable.12 Such data indicate that enterotype states are associated with long-term diet.
When Prof Jutel investigated the gut microbiome for patients from different European areas, he was able to predict whether the subject was obese or not, and found that different asthma endotypes also correlated with microbiome composition (unpublished data).
Ultimately, improved understanding of pathophysiology of allergic diseases in individual patients, said Prof Jutel, should make it possible to design better vaccines. At FASIT, Carsten Schmidt-Weber reviewed adjuvants and immune modulators considered attractive prospects for AIT. These included chitin-based adjuvants (engaging mitochondrial stress pathway), mRNA vaccinations, vaccination-driven antibody responses dependent on the microbiome, functional hydrogels and ‘doped’ nanoparticles, and JAK kinase inhibitors.
When patients are found to be non-responders, Prof Jutel concluded, a new approach could be taken to analyse phenotypes to consider whether any additional factors (such as obesity or the microbiome) might be affecting immunological mechanisms.
Molecular and Other Approaches for Allergen Immunotherapy: The Peril of Phase III Trials
Professor Jörg Kleine-Tebbe
For AIT, said Prof Kleine-Tebbe, some successful Phase II trials have failed to translate into positive Phase III trials. First, he considered evidence for T cell epitope trials. It was Phil Norman, he said, who first showed T-cell reactive treatment peptides (using two peptides of 27 amino acids from major cat allergen Fel d 1) improved human allergic responses to cats.13 The concept was taken forward by Circassia Pharmaceuticals, plc. (Oxford, UK), who developed Cat-SPIRE®, a new class of synthetic peptide immune-regulatory epitopes (involving seven synthetic peptide T-cell epitopes with short sequences derived from Fel d 1). A study of Cat-SPIRE demonstrated improvements in the primary endpoint (total rhinoconjunctivitis symptoms) after both 1 and 2 years of treatment.
For the study, subjects were exposed to cat allergens in environmental exposure chambers before and after treatment with Cat-SPIRE delivered intradermally over 3 months.14,15 However, in the following Phase III Cat-SPIRE field study, involving 1,409 cat-allergic subjects, Cat-SPIRE did not meet the primary endpoint (a combined score of symptoms and allergy medications).16 Such data illustrate how one of the biggest hurdles for AIT treatments, said Prof Kleine-Tebbe, is the transition from Phase II to Phase III studies. A key difference is that Phase II studies might take place in environmental exposure chambers or employ various in vivo-challenge models, while Phase III studies take place in the field.
Notably for Cat-SPIRE, said Prof Kleine-Tebbe, there was a considerable placebo effect (58.5%), which set a high bar for any therapeutic intervention. A range of factors, he explained, contributed to placebo effects, including regression to the mean, the Hawthorne effect (which could mean that observing patients in itself changes the outcome), variable exposures, and subjective expectations.
A recent publication argued that placebo effects in acute pain and a range of clinical conditions activate brain regions associated with self-generated emotion, self-evaluation, thinking about the future, social cognition, and valuation of rewards and punishment.17 A conjugated Amb a 1–toll-like receptor 9 agonist vaccine (TolambaTM) for ragweed-allergic subjects suffering from rhinitis also failed to translate from Phase II to III trials.
In the Phase II trial, the AIT vaccine (compared to placebo) reduced allergic rhinitis symptoms during the 2001 ragweed season (peak season, p=0.006) and maintained effects during the 2002 ragweed season (peak season, p=0.02).18 However, the subsequent Phase II/III trial involving 738 ragweed allergic subjects failed to show an effect over placebo.19 Also, in some instances where early studies have proved positive there was no further development, including recombinant birch pollen major allergen Bet v 1-vaccine and a mixture of five recombinant grass pollen allergens.20,21 Considering evidence for the recombinant B cell epitope based vaccines, Prof Kleine-Tebbe said that less studies have been undertaken than for T cell epitopes. One randomised double-blind placebo controlled AIT study in a chamber showed a small decrease in nasal and ocular symptoms and titrated skin prick testing in comparison to placebo.22 Field trials are planned.
Potential reasons for negative results in AIT Phase II/III trials, said Prof Kleine-Tebbe, include low pollen counts and selecting the wrong patients for the study. For example, the inconclusive results obtained in a study of grass SCIT by Prof Kleine-Tebbe were attributed to very low grass pollen count that season.23 In another study, subjects showed considerable pre-seasonal symptoms and no increase during the main grass season suggesting symptoms reported were not primarily reflecting grass pollen exposure.24 Such studies raise questions around how far Phase III trials can be considered reliable or to truly represent real life situations. That is not to say that there have not been AIT treatments shown to be positive in both Phase II and III trials. One example is SQ-standardised grass allergy immunotherapy with SLIT tablets, which was found to be effective after 1 year of treatment,25 and after 3 years of treatment and a further 2 years of follow-up.26
Summarising his data, Prof Kleine-Tebbe said factors influencing results include high placebo effects, the natural variability of the environment (i.e. unpredictable pollen seasons), whether field studies represent the real world, patient heterogeneity, and the use of different endpoints in Phase II and Phase III trials. The FASIT delegates had identified steps to improve the design of trials, including introduction of a standardisation approach where patients are exposed to allergens in chambers to distinguish allergic from sensitised patients, removal of high placebo responding patients (if that is ethically valid), and introducing the same efficacy parameters in Phase II and III trials. Individual patient data and presentation of results in more detail would provide valuable information beyond simply reporting primary endpoint results.
With Phase III trials, Prof Kleine-Tebbe said, it was only when “every wheel” of the complex “study machinery” moved in the same direction that positive results could be achieved.
The Use of Allergen Immunotherapy in Europe: Regulatory Aspects and Call for Harmonisation
Professor Oliver Pfaar
Prof Pfaar considered two issues debated at FASIT: i) whether AEC were ready for Phase III trials, and ii) whether it was realistic to conduct childrens’ studies in the face of PIP?
In 2008, the EMA provided guidelines for the clinical development of AIT products for allergic diseases. The guidelines stated that clinical efficacy in Phase III (pivotal) trials must be demonstrated under natural exposure of the respective allergens which evoke symptoms.27 However, such a nature-based approach has methodological problems, with Prof Pfaar outlining examples of the unpredictability of natural allergen exposure. The high variability of pollen exposure is illustrated by a study comparing pollen counts in Paris (France) and Copenhagen (Denmark) in 2007 and 2008.27 The results showed that pollen seasons start earlier in Paris than Copenhagen, and additionally that there were striking variations of pollen levels in Paris between the same months in 2007 and 2008. The consequence, said Prof Pfaar, is that findings would most likely be very different for trials conducted in Paris and Copenhagen and in different years. Another example is a post hoc analysis of data collected over six trials and six grass pollen seasons by Durham et al.,28 which showed that the magnitude of the treatment effects of grass SLIT were greater for higher pollen exposures.28 In another study, the HIALINE working group29 found that allergen release per pollen (potency) varied substantially, ranging from <1–9 pg of Phl p 5 per pollen. Such variation was in addition to natural variations in pollen count. The bottom line, said Prof Pfaar, is that nature is beyond our control and allergen exposure cannot be predicted or controlled. But attempts have been made to ‘control nature’. In the 1980s, Prof Friedrich Horak developed the first Allergy Provocation Chamber (the Vienna Challenge Chamber) to improve controllability of allergen exposure. Over the years, several AEC have been introduced worldwide, with designs ranging from fixed chambers with large capacities, to mobile chambers.
Advantages of chambers include the possibility to expose patients to defined levels of allergen that control factors including particles/m3, the temperature, air-pressure, and humidity. Other advantages include the absence of confounding allergens, no bias rescue-medication intake, no impact of personal context (outdoor versus indoor activities), and easy collection of biological samples. Additionally, lower numbers of subjects are needed than in field trials, which has an ethical benefit and results in cost savings.27,30
EMA guidelines state that AEC (as with other challenge methods) can be used for analysing primary endpoints in Phase II studies, such as dose range findings, ‘proof-of-concept’, or ‘onset of action’ trials.
But for pivotal Phase III trials, the guidelines emphasise that: “Provocation tests in allergen chambers are deemed to be promising tools for the evaluation of efficacy, however the results of such provocations have to be validated in comparison with clinical symptoms by natural exposure before such tests could be used as primary endpoints.”31 However, Prof Pfaar added, no clear further guidance about this validation procedure is given in the EMA guideline. The EAACI therefore recently set up a task force initiative bringing together academics, regulators, and chamber operators to consider technical prerequisites, model validation, and comparability with field trials.32
Regarding technical prerequisites, the group recommended validation of the allergenic exposure (including at least pollen grain counts and distribution), the use of GMP-grade allergens when processed material is mandated by local regulatory authorities, control of the chamber environment, standard operating procedures for cleaning facilities after each exposure, standard operating procedures for chamber operation, and logging of data for each exposure session.
For model validation, the group considered there should be evaluation of the outcomes of the plateau phase of the challenge, justification of single versus serial challenges, justification of low versus high dose challenges, disclosure of general patient selection criteria to assure appropriate patient selection and safety, use of standardised and clinically relevant outcomes, and reporting and justification of the calculation of the effect size of the intervention.
For comparability of allergen chamber trials with field trials, the group considered that direct comparison was not possible as natural allergen exposure cannot be determined and rescue medication is allowed in field trials. Indirect comparisons of effect size, however, are possible when allergic subjects with comparable clinical characteristics are evaluated. Most importantly, the panel concluded that hybrid trial designs that correlate AIT treatment effect sizes (demonstrated under natural exposure during the season) with those demonstrated under AEC challenges in the same patient population are feasible and may represent an option.
Looking into his crystal ball, Prof Pfaar foresaw a future where clinical validation chambers would be used in Phase III trials. For this vision to be realised, collaboration between chamber operators, academics, and manufacturers of AIT products will be needed.
The EMA/PDCO (Paediatric Committee) Standard Paediatric Investigation plan for Allergen Products for Specific Immunotherapy (Revision 4), which came into force in 2009, is now mandatory before companies submit marketing authorisation.33 The PIP specified that separate dose-finding AIT studies are not required in children, but proof of long-term efficacy is.
Marketing authorisation applicants have to select one allergen product of their portfolio which will be evaluated for long-term disease modifying efficacy in adults and children (3-year treatment and 2-year post-treatment follow-up). Once long-term efficacy of the selected product has been established in adults and children, development of all other products will depend on the proposed marketing authorisation application (treatment of allergic symptoms, sustained clinical effect, and long-term efficacy/disease modifying effect).
Prof Pfaar reviewed the FASIT debate around whether long-term efficacy studies are really realistic for children, which at the FASIT meeting was moderated by Prof Matthias Kopp. He gave the example of the GAP trial by Erkka Valovirta, where, after 7 months of screening, recruitment of 812 children at 101 centres in 11 countries was achieved.34 While a “big job with a heavy work load”, said Prof Pfaar, the GAP study does in principle demonstrate the feasibility of PIP studies in SLIT. There has, however, been no single published trial for SCIT meeting the PDCO criteria. The FASIT panel assessed the co-operation of ethical committees in approving SCIT trials and concluded that the willingness of parents to enter their children into 5-year placebo controlled SCIT studies was low.
Long-term studies in children, said Prof Pfaar, were needed but raised issues over whether it was ethical to deprive children (in the placebo arm) of known treatment benefits for long periods of time. Therefore, he considered what should be viewed as the best ‘realistic’ grade of evidence for AIT clinical efficacy in the paediatric population. Many unresolved issues exist, including ideal clinical endpoints and whether biomarkers might be used. Currently, Prof Pfaar concluded, there is a kind of ‘paralysis’/reluctance among researchers, manufacturers, experts, and authorities with regard to designing proper clinical studies in children. Prof Pfaar ended his presentation with an urgent call for further consultation between all parties involved in AIT.
How Can we Offer High Quality Diagnostics for Our Patients in the Future?
Professor Piotr Kuna
One of the major issues discussed at FASIT 2017, said Prof Kuna, was the threat facing diagnostics from EU legislation. The consequences, he said, placed the entire medical discipline of allergology (allergy) in peril. During the FASIT meeting, Prof Torsten Zuberbier and Prof Ludger Klimek gave an overview on the current situation.
The situation was due to the consequences of the EU directive 2001/A3/ EC article 1 4b, which states allergens in general should be considered as drugs, falling under relevant national and European legislation.35 Unfortunately, the directive made no distinction between allergens used for therapeutic and diagnostic purposes. The bottom line, said Prof Kuna, was that allergens used for diagnosis need to obtain marketing authorisation, and are required to demonstrate safety, sensitivity, and specificity in clinical trials. Furthermore, during routine clinical use, regular dossier updates are required showing handling of variation processes, ongoing stability testing, and periodic safety update reports.35,36
Such legislation, he added, will apply to a range of diagnostics including prick tests, prick to prick tests, intracutaneous tests, nasal provocation tests, challenge tests (e.g. food), and patch tests. Skin provocation tests, he said, represent the cornerstone of the diagnosis of Type 1 allergies. They are safe, fast, and inexpensive, and furthermore clinicians have had decades of experience using them. Modern laboratory allergy diagnosis can be considered to add to in vivo tests, but do not replace them. An online survey of an EAACI Task Force led by Vicky Cardona and Ludger Klimek, conducted in 2017, questioning representatives from 31 national EAACI-affiliated societies about allergy diagnosis, demonstrated skin tests are preferred to in vitro tests. Results showed that 90.3% of respondents preferred skin tests for respiratory allergy, 64.5% for food allergy, 58.1% for insect venom allergy, 54.1% for atopic dermatitis, and 58.1% for urticaria.37
Already, consequences from the legislation are being observed. Since 2004 France has lost twothirds of prick test diagnostics; in 2013 alone, Germany lost 443 authorised diagnostics; and, in the Netherlands, only six authorised diagnostic tests are currently available.38 Estimates for the German Health system, suggest totally replacing allergy diagnosis with laboratory diagnosis (such as basophil activation tests) would increase costs by a factor of 3.5 to 5.38
Obtaining marketing authorisation for in vivo tests is also likely to be expensive. Estimates taking into
account clinical development costs, Phase I, II, and III trials and PIP testing, suggest the total cost
per skin prick registration would be a minimum of €385,000. Such expenditure would result in street
prices of €1,500 for rare allergens and >€60 for more frequently used allergens.39 To ensure continued availability of in vivo allergy diagnostics, said Prof Kuna, all stakeholders (EAACI, manufacturers, the EMA, and national regulators) need to come together to resolve the issues. The EAACI has established a task force to consider the issue of allergy diagnostics and is approaching the EU commission in an attempt to change European legislation.
The EAACI has also proposed that manufacturers should consider ‘exchanging’ rare allergens so that
they are not all faced with supporting all allergens in all markets. Without in vivo allergen diagnostic testing, Prof Kuna concluded, the allergy community will not exist. Either amendment to the current legislation or the introduction of new legislation, he said, will be needed to save the discipline.
Conclusion
The FASIT meetings, said Prof Wahn, ensure conversations do not remain as just academic discussions in ivory towers, but are shared widely throughout the world. The aim, he said, was to develop the best possible immunotherapy for patients and ensure a future for both patients and allergists. He gave credit to Allergopharma for providing a platform to enable speakers to address important issues and discuss the future of allergology.