Chairpersons: Markus Magerl (symposium 1),1 Pavlína Králíčková (symposium 2)2
1. Department of Dermatology and Allergy, Charite Universitätsmedizin, Berlin, Germany
2. Department of Clinical Immunology and Allergology, University Hospital Hradec Králové, Czechia
Speakers (Symposium 1): Grzegorz Porębski,1 Mauro Cancian2
Speakers (Symposium 2):Emel Aygören-Pürsün,3 Teresa Caballero4
1. Department of Clinical and Environmental Allergology, Jagiellonian University Medical College, Kraków, Poland
2. Department of Systems Medicine, University of Padova, Italy
3. Angioedema Clinic and Center for Hereditary Angioedema, University Hospital Frankfurt, Goethe University, Germany
4. Allergy Department, Hospital Universitario La Paz, Madrid, Spain
Disclosure: Magerl has received research grant support and speaker/consultancy fees from BioCryst Pharmaceuticals, CSL Behring, Jerini/Dyax/Shire/Takeda Pharmaceuticals, Kalvista Pharmaceuticals, Pharming Group NV, and Octapharma. Králíčková has received honoraria as a consultant and speaker for Takeda and CSL Behring; and symposium sponsorship from Takeda and CSL Behring. Porębski has received honoraria as a speaker, advisory board member, or clinical trials investigator from BioCryst Pharmaceuticals, CSL Behring, Pharming Group NV, and Takeda Pharmaceuticals; and as a committee member for Novo Nordisk. Cancian has received grant research support and honoraria as a speaker or consultant for BioCryst Pharmaceuticals, CSL Behring, Kalvista, Pharming Group NV, Shire/Takeda Pharmaceuticals, SOBI, and UCB; and clinical trial/registry investigator support from BioCryst Pharmaceuticals, CSL Behring, Kalvista, Novartis, Pharming Group NV, Shire/Takeda Pharmaceuticals, SOBI, and UCB. Aygören-Pürsün has received honoraria as an advisor for BioCryst Pharmaceuticals, Biomarin, CSL Behring, Kalvista, Pharming Group NV/SOBI, Pharvaris, and Shire/Takeda Pharmaceuticals; for clinical trials from BioCryst Pharmaceuticals, CSL Behring, Kalvista, Pharvaris, and Shire/Takeda Pharmaceuticals; as a speaker for CSL Behring, Centogene, Pharming Group NV, and Shire/Takeda Pharmaceuticals; and grants from CSL Behring and Shire/Takeda Pharmaceuticals. Caballero has received honoraria for advisory boards or clinical studies/registries from BioCryst Pharmaceuticals, CSL Behring, Novartis, Pharming Group NV, and Takeda Pharmaceuticals; as a speaker for CSL Behring, Novartis, Pharming Group NV, and Takeda Pharmaceuticals; as a consultant for BioCryst Pharmaceuticals, CSL Behring, Novartis, Octapharma, Pharming Group NV, and Takeda Pharmaceuticals; has received research funding from AEDAF, CSL Behring, and Takeda Pharmaceuticals; and has provided writing support to BioCryst Pharmaceuticals, CSL Behring, and Takeda Pharmaceuticals.
Acknowledgements: Medical writing assistance was provided by Eleanor Roberts, Beeline Science Communications, Ltd, London, UK, and supported by Hannah Moir, Senior Medical Writer, EMJ, London, UK.
Support: The publication of this article was funded and reviewed by CSL Behring. The views and opinions expressed are those of the speakers and not necessarily of CSL Behring.
Citation: EMJ Allergy Immunol. 2022;7[1]:42-51. DOI/10.33590/emjallergyimmunol/10166350. https://doi.org/10.33590/emjallergyimmunol/10166350.
Meeting Summary
A two-part digital symposium series entitled ‘Hereditary Angioedema (HAE) Management: From Dealing to Leading,’ took place during the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress, held in Prague, Czechia, in July 2022. The first symposium, ‘The Journey Towards Disease Control in HAE’, held on 1st July 2022, was chaired by Markus Magerl, Department of Dermatology and Allergy, Charite Universitätsmedizin, Berlin, Germany. Speakers Grzegorz Porębski, Department of Clinical and Environmental Allergology, Jagiellonian University Medical College, Kraków, Poland, and Mauro Cancian, Department of Systems Medicine, University of Padova, Italy, discussed how the advent of new disease-specific HAE treatments have contributed to the evolution of the HAE management guidelines, and the resulting impact on the lives of patients with HAE. The latest international management guidelines from the World Allergy Organization (WAO)/EAACI newly define the goals of treatment in HAE as achieving total control of the disease and normalising patients’ lives, stressing that this can currently only be achieved by long-term prophylactic (LTP) treatment. The second symposium, ‘Making the Goals of HAE Management Achievable with Subcutaneous C1-Inhibitor’, held on 2nd July 2022, was chaired by Pavlína Králíčková, Department of Clinical Immunology and Allergology, University Hospital Hradec Králové, Czechia, who also provided a brief overview of the development of the subcutaneous formulation of C1-inhibitor for LTP. Emel Aygören-Pürsün, Angioedema Clinic and Center for Hereditary Angioedema, University Hospital Frankfurt, Goethe University, Germany, and Teresa Caballero, Allergy Department, Hospital Universitario La Paz, Madrid, Spain, then used case studies to highlight the necessity of assessing and monitoring a patient’s disease activity, the associated quality of life, and disease control to allow for possible adaptations to the treatment plan. Both speakers also highlighted how the use of subcutaneous C1-inhibitor for LTP can contribute towards the achievement of the goals of HAE treatment, namely achieving total disease control and normalising patients’ lives.
INTRODUCTION
Hereditary angioedema (HAE) is a rare genetic disease that manifests as recurrent cutaneous or submucosal oedema, most commonly affecting the skin, the abdomen, and upper respiratory tract.1 Pain, disfigurement, nausea, and fatigue can all be experienced during an HAE attack,2-4 with manifestation, frequency, and severity varying both between patients and within the same patient, making HAE an unpredictable condition.1 The unpredictability of HAE can cause substantial physical and emotional impairment, at the time of an attack but also between attacks, potentially due to the continuous fear of attacks, the need to avoid triggers of attacks, the psychological distress due to the chronic disease burden, and the presence of comorbid diseases such as depression and anxiety.3,5-7
HAE has an estimated prevalence of 1:50,000 in the population with onset of symptoms typically occurring in childhood or adolescence.1 HAE Type 1 and 2 are caused by mutations in the gene SERPING1, which codes for the serine protease C1-esterase inhibitor (C1-INH).8 In patients with HAE Type 1, both C1-INH protein levels and function are low; in those with HAE Type 2, C1-INH protein levels are either normal or elevated but C1-INH function is low.1
C1-INH acts as the major inhibitor of the complement proteases C1r and C1s and mannose-binding lectin-associated serine proteases (MASP) 1 and 2, as well as the contact-system proteases plasma kallikrein and coagulation factor XIIa. Additionally, C1-INH plays a minor role in the inhibition of plasmin and factor XIa in the fibrinolytic and coagulation systems, respectively. In the absence of sufficient functional C1-INH levels, the activation of these target proteases is enhanced. In HAE Types 1 and 2, the deficiency of functional C1-INH leads to uncontrolled activation of plasma kallikrein and FXII, in turn leading to the overproduction of bradykinin, which, upon binding to the bradykinin B2 receptor, results in increased vascular permeability and swelling.1,9,10
SYMPOSIUM 1: THE JOURNEY TOWARDS DISEASE CONTROL IN HEREDITARY ANGIOEDEMA
Day 1 of the two-day symposium series focused on the goals of treatment in HAE. Porębski presented a talk entitled ‘HAE Then and Now: Evolving Treatment Goals’, in which he summarised the important milestones in the development of HAE management guidelines and highlighted the new treatment goals. Cancian then followed with a discussion on the ‘Impact of the New HAE Guidelines on Patients’, to demonstrate the practical implications for patients resulting from the implementation of treatment guidelines in daily practice.
To establish how the management guidelines of HAE have evolved over time due to the availability of new therapeutic options, Porębski and Cancian both provided an overview of the milestones in the treatment of HAE. The treatment of HAE Types 1 and 2 is based on three pillars: on-demand or acute treatment (aiming to control HAE attacks when they occur); short-term prophylaxis ([STP]; aiming to prevent HAE attacks during exposure situations with an increased risk of an attack, including preprocedural prophylaxis); and long-term prophylaxis ([LTP]; routine treatment to reduce the burden of HAE by preventing attacks).1 As stressed by Cancian, these treatment modalities “should not be regarded as mutually exclusive, but rather as additional opportunities to be used in the same patient.” Whereas attenuated androgens, antifibrinolytics, and fresh frozen plasma were being used for the management of HAE in the 1960s, the discovery of the role of C1-INH and bradykinin in the pathogenesis of HAE opened the way for disease-specific treatments targeting different levels within the contact system. While the 1970s saw the introduction of purified plasma-derived C1-INH replacement therapy, the first consensus algorithms for HAE management did not appear until 2004.2,11 Additional disease-specific treatments with novel mechanisms of action were introduced early in the 21st century for the treatment of acute HAE attacks, including recombinant C1-INH, bradykinin antagonist icatibant, and kallikrein inhibitor ecallantide, leading to corresponding updates in consensus treatment algorithms.12 During the last decade, a number of highly effective and safe disease-specific treatment options for LTP, including intravenous (IV) and subcutaneous (SC) C1-INH and the kallikrein inhibitors lanadelumab and berotralstat, have further contributed to the advancement of the HAE management guidelines.1,13,14
The 2021 revision and update of the WAO/EAACI guideline for the management of HAE was developed with the contribution of international experts (including clinicians, scientists, patients with HAE, and patient advocates) from different countries spanning five continents, using the Delphi method. Porębski highlighted the main changes that have been implemented, the most important of which is the inclusion of the newly defined ultimate goals for the treatment of HAE: achieving total control of the disease and normalising patients’ lives.1 Providing additional insight into the ultimate goals, the guideline clarifies that they essentially translate to patients no longer having attacks, which can currently “only be achieved by LTP treatment.” The recommended first-line treatment options for LTP now include SC and IV plasma-derived C1-INH, lanadelumab, and berotralstat, and it is the availability of these modern treatment options of personalised disease management, and of new tools for measuring treatment outcomes that make achieving complete control of HAE a realistic possibility for many patients.1
Over the years, new evidence on the burden of HAE, and heightened physician awareness of the burden, had implications on the treatment goals in HAE, especially with respect to LTP. Porębski presented his personal experience from a study conducted in 2009, in which >50% of patients reported moderate-to-extreme impact of HAE on many aspects of their lives including travel plans, free time, concern about their appearance, social life, and a sense of responsibility about transmitting HAE to any offspring.15 The understanding that the impact of HAE is much more than just the attacks experienced brought with it a greater understanding of the importance of LTP and an evolution in the criteria for initiating LTP. There is now agreement that the decision to start a patient on LTP should not be based on attack frequency and severity alone, but should be individualised taking into consideration disease activity, burden, and control, as well as the patient’s preference.1,16 Access to urgent care and the benefit–risk profile and treatment burden of available acute and prophylaxis therapies are also factors to consider.16 Importantly, initiation of LTP should be a joint decision between the patient and clinician17 and evaluation of the need for LTP should be carried out at each clinic visit.1
Porębski also highlighted the update that was made in the recommendation for STP; whereas previously STP had been recommended before procedures that can induce an attack, the new guideline now also suggests considering STP prior to exposure to patient-specific angioedema-inducing situations.1 The WAO/EAACI 2021 guideline-recommended HAE treatments within each treatment setting are illustrated in Table 1.
Important considerations for the future, as highlighted by Porębski, include determining whether STP should be handled differently in patients with a complete response to LTP, and whether patients initiated on LTP should continue for the duration of their lives. Additionally, knowing that treatment responses vary between individual patients, the possibility of identifying the best responders in advance would be advantageous for the implementation of precision medicine to match patients to the therapies most appropriate for them.18,19 With respect to future treatments, Porębski highlighted that 10 of the current 12 investigational drugs for HAE are being investigated for use in LTP, which aligns with the new treatment goals as defined in the 2021 WAO/EAACI guideline.1,20
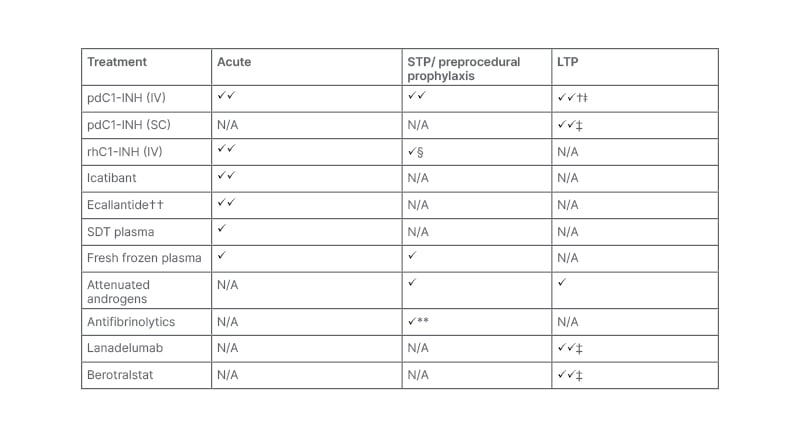
Table 1: Treatment for hereditary angioedema according to WAO/EAACI 2021 guidelines.1*
*Guideline recommendations may vary from the approved product indications across different countries. üü indicates the first-line recommendations; ü indicates alternative treatment options when first-line options are not available; † indication for use varies by manufacturer; ǂ in patients ≥6 years old; ‡ in patients ≥12 years old; § could be considered if IV pdC1-INH is not available; ** not recommended by most guideline experts; †† currently approved in the USA only.
EAACI: European Academy of Allergy and Clinical Immunology; IV: intravenous; LTP: long-term prophylaxis; N/A: not applicable; pdC1-INH: plasma-derived C1-inhibitor; rhC1-INH: recombinant human C1-inhibitor; SC: subcutaneous; SDT: solvent detergent-treated; STP: short-term prophylaxis; WAO: World Allergy Organization.
Cancian then elaborated on the impact of the new guidelines on patients, stressing that the new goals of treatment reflect a changing treatment paradigm and the vast progress that has been made compared to the earliest consensus algorithms and guidelines: within the last two decades, the aim of treatment in HAE has moved significantly beyond just reducing the risk of mortality from acute attacks to also encompass reducing the overall burden of the disease on patients. Burden of disease is also relevant for paediatric patients and their parents, and specialised guidelines for paediatric patients have now been developed.21
Discussing the heightened awareness around the burden of HAE, Cancian underscored the importance of the patient-reported outcome measures (PRO) that have been validated in recent years to better understand the global burden of angioedema from the patient’s perspective. There is now consensus agreement among the HAE community that patients with HAE should provide input on how they or their treating physicians assess whether HAE is controlled or their life is normalised.17 For the first time, the 2021 WAO/EAACI guideline puts particular emphasis on the importance of patients monitoring their disease activity, impact, and control to optimise treatment, particularly in patients who are using LTP.1 As detailed in Table 2, there are a number of generic and HAE-specific tools that can help with this.6 Cancian particularly emphasised the attention that has been given to the development of questionnaires to assess the quality of life (QoL) of patients. Giving his own personal experience, he mentioned that in the past his patients would be asked to document their attack frequency, location, and whether the attacks had been treated, but only to prescribe new medication. In current practice, time is taken to have qualitative discussions with patients to understand how they feel in general and whether their current therapy is effectively reducing the burden of disease and improving QoL.
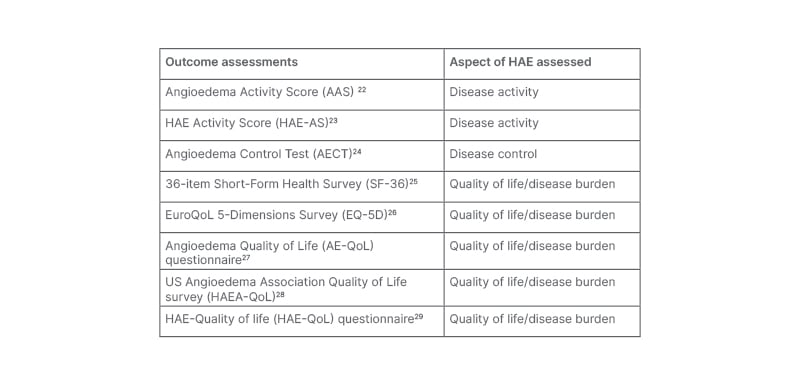
Table 2: Patient-reported outcome measures in hereditary angioedema.
HAE: hereditary angioedema.
Cancian stressed that real-world application of guidelines is not always easy, particularly in rare diseases. It necessitates close collaboration between reference centres, clinical and scientific networks, patient associations, single physicians, and their patients. Of note, disparities in healthcare resources for the management of HAE among different countries also still need to be resolved.30
In the closing discussion of the symposium, the speakers addressed key questions and firstly focused on how often a patient’s progress should be monitored using PROs. There was agreement that this should generally be done at each visit, but individualisation is also needed based on disease severity. Magerl mentioned that, particularly when there is a change to a treatment plan, it may be necessary to monitor more frequently.
With regard to the integration of the guidelines into daily practice, Porębski stressed the importance of maintaining close collaboration with patients and of increasing their awareness of the guidelines, ensuring patients know the implications for their treatment and QoL.
The issue of how long a patient should remain on LTP was discussed, with Cancian suggesting that, given that patient phenotype changes over time, following the first year, LTP could be periodically suspended to ascertain if it is still needed, again using an individualised approach. Porębski commented that the best situation would be to eventually have a biomarker.
SYMPOSIUM 2: MAKING THE GOALS OF HEREDITARY ANGIOEDEMA MANAGEMENT ACHIEVABLE WITH SUBCUTANEOUS C1-ESTERASE INHIBITOR
The second day of the symposium series looked at how SC C1-INH, as a new LTP option, can help clinicians and patients achieve total disease control and normalisation of patients’ lives. Králíčková opened with a brief introduction to SC C1-INH, followed by Aygören-Pürsün who explained the multi-dimensional impairment that patients with HAE experience in her presentation ‘Managing HAE: More Than Just Treating Attacks’. Caballero then discussed ‘Optimising Long-Term Prophylaxis: Treating-to-Target Made Possible’, focusing on the need for continuous reassessment of patients’ treatment plans in order to determine the extent to which the treatment goals are being achieved and to make adjustments as appropriate. Aygören-Pürsün and Caballero presented examples of their own patient cases to demonstrate how SC C1-INH can help patients achieve adequate disease control, improving patients’ QoL and enabling them to live normal lives.
Regular IV C1-INH replacement, administered twice weekly, has been shown to be an effective treatment option for LTP with an acceptable safety profile, and has thus had a first-line recommendation in HAE management guidelines for several years. To overcome the administration burden associated with twice weekly regular IV infusions, a SC C1-INH formulation for LTP was developed.31 The SC route can provide greater convenience in administration as well improved steady-state plasma concentrations of C1-INH compared with LTP with IV C1-INH, thus allowing for better symptom control.1,32,33 Králíčková highlighted results from the randomised, double-blind, placebo-controlled Phase III study of SC C1-INH (COMPACT), in which the 60 IU/kg twice weekly dose of SC C1-INH led to a 95% median reduction in attacks versus placebo and a 100% median reduction in the use of rescue medication.31 In the open-label, parallel-arm extension study of COMPACT, patients receiving 60 IU/kg SC C1-INH (n=63) achieved a median annualised attack rate of 1.0 attack per year.34 Králíčková further emphasised that these patients also experienced clinically meaningful and sustained improvements from baseline in overall QoL, anxiety, depression, productivity, and satisfaction with therapy.35 Adverse events with SC C1-INH were reported in similar proportions of patients as compared with placebo and predominantly included injection-site reactions, though these occurred at a low rate, were mild, and resolved within 24 hours. No anaphylactic reactions or neutralising antibodies to C1-INH were observed.31,34 Studies in adolescent,36 elderly,37 and female38 patients have demonstrated similar efficacy and tolerability.
Building upon the disease burden discussion of the first symposium, Aygören-Pürsün emphasised that the burden of HAE is not just the symptoms suffered during an attack. Patients experience multi-dimensional impairment in QoL, including comorbid anxiety and depression, time lost from social activities, school, and work, loss in productivity, and the burden placed on caregivers.5-7 Patients’ anxiety levels have been demonstrated to be in direct association with the level of pain experienced during their most recent attack.5 Presenting data from a recent multinational patient survey examining the burden of illness in patients with HAE, Aygören-Pürsün explained that attack frequency and the severity of anxiety and depression are determinants of QoL and disease control for patients with HAE. As a result of the frequency of their attacks as well as their anxiety and depression severity, the majority of patients with HAE within the survey (82%) demonstrated poor disease control, with Angioedema Control Test (AECT) scores of <10.3
HAE can also potentially have a negative impact on educational and career opportunities of patients. Showing data from analyses performed in Europe, Aygören-Pürsün emphasised that patients miss an average of 20 days of school or work per year, and 45% of patients were absent from school or work during their last attack, which reaches 80% for a severe attack.39,40
Aygören-Pürsün went on to illustrate how the use of SC C1-INH can positively impact a patient’s QoL, by presenting the case of a 41-year-old female patient with comorbid anxiety disorder and autoimmune thyroiditis. The patient’s initial treatment was on-demand only, with IV C1-INH or SC icatibant; however, the patient experienced uncontrolled disease (rated by the AECT), attacks every 2−4 days, and other effects to their general health and QoL. Though initially reluctant to use LTP, the patient was prescribed SC C1-INH when they were 39-years-old, taking into account both their attack frequency and anxiety regarding the attacks. Though they did have some mild attacks during the initial transitional period and during a period of extreme stress related to personal circumstances, the patient experienced a 7-month long attack-free period. As Aygören-Pürsün underscored, the impact of the attack reduction was also reflected in the AECT measuring disease control which improved from 5 out of 16 (poor control) to 15 out of 16 (nearly complete control). The patient also showed increased scores on both general health and QoL measures, including diminished attack-induced anxiety.
Reflecting on this case example, Aygören-Pürsün shared how her patient’s experience also resonates with a recent survey of 14 patients in the USA that received LTP with SC C1-INH for at least 3 months. Within this survey, patients reported improved QoL across multiple domains; importantly, patients reported no longer feeling limited by HAE and having less HAE-related anxiety and depression, expressing increased feelings of confidence, independence, optimism, and normalcy.41 Aygören-Pürsün summarised the key implications for treatment decisions in clinical practice, reiterating that many patients with HAE, despite effective treatment of their attacks, continue to have multi-dimensional impairment in their QoL. Therefore, management of HAE should include regular assessment of QoL and disease control as the basis for treatment decisions.
The need for continuous monitoring of HAE disease activity, impact, and control was further underscored by Caballero as she presented a detailed case of a male paediatric patient and introduced the factors that should be considered when assessing whether a treatment plan is achieving disease control and normalisation of patients’ lives. These factors enable a ‘treat-to-target’ approach for optimisation of HAE treatment and were agreed upon in the Delphi consensus process which led to the updated 2021 WAO/EAACI guideline (Table 3).1,17
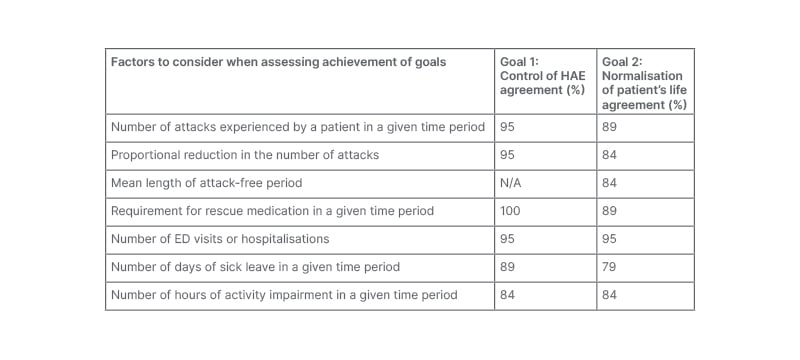
Table 3: Treating to target in hereditary angioedema. Consensus statements on the factors to consider when assessing disease control and normalisation of the patient’s life.17
ED: emergency department; HAE: hereditary angioedema; N/A: not applicable.
The male patient was diagnosed aged 3 months during a family screening protocol, before they had exhibited angioedema symptoms. The treatment plan agreed at diagnosis was on-demand treatment and pre-procedural prophylaxis, as needed, with IV pdC1-INH. Their first attack, at age 2 years, resulted in an emergency department visit. By age 11, the patient’s disease activity had increased significantly and was having an impact on QoL. Both they and their parents missed time from school and work, respectively, and the patient received psychological support due to anxiety. The patient could not be autonomous or participate in sports, and teachers were concerned about their performance in school. The treatment plan was modified to receive SC icatibant for acute attacks, which they and their parents were trained to administer, with emergency department administration of IV pdC1-INH if required. They were also initiated on LTP with oral tranexamic acid.
Although at age 12 there was partial improvement in the patient’s attack rate, there continued to be unmet needs: the patient was still not participating in sports or attending extracurricular activities, they were still receiving psychological support, and their parents were still missing workdays. The treatment plan was thus updated with a switch to LTP with self-administered SC C1-INH. Within the next year, the patient was attack-free and QoL improved substantially; they did not miss any school days, require any hospitalisations or emergency department visits due to HAE, and both they and their parents were very satisfied with the treatment.
This case, Caballero concluded, highlighted the particular needs of paediatric patients and their caregivers; HAE can impact a child’s attendance and performance in school, potentially preventing future educational or career opportunities, and leads to anxiety for both the patient and caregivers.40 The case also illustrated the importance of developing treatment plans in HAE based on a ‘treat-to-target’ approach. As such, treatment efficacy and safety, as well as the psychological impact of HAE, need to be regularly assessed and treatment plans adapted in order to achieve optimal results.1,5 Caballero also emphasised that drug self-administration is feasible for young adolescents and that all patients should be trained to safely self-administer IV and SC therapies licensed for self-administration.21
In the closing remarks of the symposium, the speakers addressed key questions and discussed the advantages of SC C1-INH. Aygören-Pürsün remarked how SC C1-INH use by her patients has led to vast reductions in HAE attacks and long attack-free periods, giving patients more opportunity to live the life they always wanted to live. With respect to monitoring progress after initiating LTP with SC C1-INH, Aygören-Pürsün also remarked that disease control and QoL questionnaires are completed by patients at every visit, so that a picture of their progress can be gained at the start of a consultation.
When discussing the treatment of adolescent patients, the importance of working with patients and their caregivers to ensure adherence to the treatment plan was emphasised, with Caballero remarking that using QoL instruments is particularly important to gain insight into specific problems the adolescent may be facing and to help individualise treatment.
Caballero also commented that she advises caregivers to help and encourage patients with HAE to try to live as normal a life as possible, not avoiding situations such as participating in sport due to fear of attacks, so that patients with HAE ‘live as any other person’.
CONCLUSION
With the introduction of new therapeutic options and treatment modalities, HAE management has evolved from treating attacks with the aim of reducing mortality risk to preventing attacks with the aim of reducing QoL impairments. This is reflected in the most recently published 2021 WAO/EAACI HAE management guideline, where the ultimate goals of treatment have been defined as achieving total control of the disease and normalising patients’ lives.
New options for long-term prophylaxis, such as SC C1-INH, together with personalised disease management that is optimised with the use of PROs, can ensure that patients are no longer just ‘dealing’ with their disease, but are instead ‘leading’ their disease to be able to live normal lives.






