Meeting Summary
This symposium provided an overview of past, current, and future therapies and routes of administration for patients with hereditary angioedema (HAE). Prof Cicardi opened the symposium by welcoming attendees and introducing the main topics of the session. Prof Magerl then focussed on treatments that are currently used for acute and prophylactic management of patients with HAE and highlighted that there is an unmet medical need in terms of better prophylactic treatment options. Prof Craig summarised the clinical evidence gathered over the last decades and shared the key findings and insights that led to our current understanding of the disease and laid the foundations for current and future treatment approaches. Prof Zuraw presented the findings from the pivotal Phase III COMPACT trial that explored the efficacy and safety of a self-administered subcutaneous (SC) nanofiltered C1-esterase inhibitor concentrate (C1-INH[SC]) for the prevention of HAE attacks.
Unmet Medical Needs in Hereditary Angioedema Management
Professor Markus Magerl
In the current treatment landscape for on-demand treatment of HAE there are a number of options, such as intravenous plasma-derived C1-INH (C1-INH[IV]), recombinant human C1-INH, icatibant (a bradykinin B2-receptor antagonist), and ecallantide (a kallikrein inhibitor). However, prophylactic treatment options are very limited, with only androgens, tranexamic acid, and plasma-derived C1-INH(IV) concentrate available. Plasma- derived C1-INH is the only therapy licenced for long-term prophylaxis in adults, adolescents, and children, and is recommended by international guidelines as first-line therapy for long-term prophylaxis of HAE.1 Androgens, such as danazol and stanozolol, are older drugs that are not approved for long-term prophylaxis of HAE in several European countries, which are still used in doses of ≤200 mg/day and 2 mg/day, respectively, to suppress HAE symptoms.
Tranexamic acid is no longer recommended as a long-term prophylactic treatment option because of a very low preventive effect.1 Clinical studies on the use of androgens for prophylactic treatment of HAE demonstrated that they can be quite effective in reducing the number of HAE attacks in some patients; however, their safety profile limits their use. The majority of patients experience adverse events (AE) during long-term use, and a substantial number of patients discontinue treatment because of side effects.2,3 Side effects of androgens include virilisation, weight gain, menstrual disorders, psychological abnormalities, headache, myalgia, and acne.4
On the use of an C1-INH(IV) concentrate for long-term prevention, interim results from a European registry of 45 patients showed that the majority of patients were on a routine regimen of a 3–4-day interval between C1-INH(IV) administration, while about one-third were on an intensified 1–2-day interval regimen or a prolonged 5–7-day interval regimen. Patients who adhered to the routine regimen (1,000 units IV every 3–4 days) had a lower breakthrough attack rate (1.5 attacks per month) compared with those on an intensified or prolonged regimen (around 3–5 attacks per month). Most breakthrough attacks occurred on the intended day of dosing, which indicates that C1-INH(IV) concentrate fell short in the prevention of attacks during the whole dosing interval.5
In conclusion, real-world evidence shows that HAE is well-controlled in most patients using the approved C1-INH(IV) dosing regimen; however, some patients require more intensive therapy due to insufficient response.
Real-world evidence has also demonstrated the challenges that exist with the IV administration of C1-INH(IV). An observational, retrospective cohort of USA healthcare claims data (covering an observation period from 2006–2014) of 521 patients with HAE and accessible health records revealed that 18 patients had been using SC ports for IV administration of HAE medication. Of the 18 patients, 10 had at least one major complication that lead to replacement or repair of their port.6,7
In summary, current treatment options for prophylaxis are very limited. The use of androgens is restricted by numerous contraindications and side effects. Real-world and clinical evidence on the only approved treatment option, C1-INH(IV) prophylaxis, indicates that many patients still experiencing breakthrough HAE attacks would probably benefit from a more flexible dosing recommendation (higher dose of C1-INH or shorter injection intervals). In patients with IV access failures, the use of SC ports is associated with a high risk of port-related complications. Therefore, there is an unmet medical need for more effective and more easily administered therapy.
Experience with C1-INH Replacement Therapy for Hereditary Angioedema Management
Professor Timothy Craig
The C1-INH protein inhibits the complement protein, C1, and blocks several pathways of the kallikrein cascade. Mutations in the SERPING1 gene coding for the C1-INH protein cause HAE Type I and II. Mutations that cause HAE Type I lead to reduced levels of C1-INH in the blood, while mutations that cause Type II result in the production of a C1-INH that functions abnormally. Without the proper levels of functional C1-INH, excess bradykinin is generated. Excessive accumulation of fluids in body tissues causes the episodes of swelling seen in individuals with HAE.8,9
Until the 1970s, the only treatment available for acute attacks of HAE was fresh frozen plasma, which had complications such as sensitisation, worsening of acute exacerbation of HAE, and increased risk of viral transmission. In 1973, a call for purified C1-INH was published10 and was answered by Pickering and Tamblin,11 and Brackertz and Kueppers.12 The response from the latter reported successful treatment of two patients with HAE with a partially purified preparation of C1-INH at a dose of 1,200 ‘inactivator units’ (produced by Behringwerke, Marburg, Germany).12 A critical level of functional C1-INH was first postulated by Späth et al.13 in an observational study of patients with HAE treated with oral prophylaxis. It was observed that attacks most frequently occurred when C1-INH antigen levels were <0.035 g/L, which corresponded to approximately 40% functional C1-INH, and attacks were absent when near-to-normal (≥0.075 g/L) C1-INH antigen levels were present. Bork and Witzke14 were among the first to describe the prophylactic use of C1-INH(IV) concentrate in two patients with HAE, who received 500 units of C1-INH(IV) every 4–5 days. Both patients experienced a marked reduction of attacks during treatment when C4 protein levels normalised and C1-INH levels increased above the critical threshold of C1-INH antigen (approximately 7 mg/dL).14
The first double-blind, placebo-controlled studies of C1-INH(IV) prophylaxis was carried out by Waytes et al.,15 whereby six patients with a history of >5 attacks within 1 year received 25 plasma units/kg of C1-INH(IV) or placebo every third day for two 17-day treatment periods. The primary endpoint was the daily symptom score (highest severity scores [0–4] recorded for each symptom [abdominal, extremities, laryngeal, or genitourinary] for a 6-hour period and averaged over four consecutive 6-hour periods). The mean total daily symptom scores were significantly lower in patients treated with C1-INH compared with placebo (p<0.001).15 C1-INH(IV) concentrate resulted in an increase in plasma levels of C1-INH to above the critical threshold (40% of normal). Following infusion, C1-INH levels fell to approximately the lower limit of normal within 24 hours but remained above baseline at 72 hours.15
A placebo-controlled crossover study compared the number of HAE attacks per month in 22 patients treated with C1-INH(IV) 1,000 international units (IU) twice-weekly or placebo. Patients treated with placebo had a mean attack rate of 4.6 per month, compared with 2.3 attacks per month in those treated with C1-INH(IV), which represents a 50% reduction in attack rate.16 In an open-label study, the efficacy of C1-INH(IV) was associated with a better preventative effect with shorter intervals between injections. A dose interval of 2–3 days between IV infusions was deemed optimal for HAE attack prevention.17
The pharmacokinetics (PK) of subcutaneous C1-INH was first studied using 1,000 IU of C1-INH (approved for IV use [Berinert, CSL Behring, Marburg, Germany]) in 24 patients with HAE. The mean relative bioavailability of functional C1-INH(SC) was about 40%.18 Based on these data, a Phase II study compared mean trough C1-INH activity in patients administered SC fixed doses of a volume-reduced C1-INH concentrate of 1,500, 3,000, and 6,000 IU. Patients who received the 1,500 IU dose achieved a mean trough C1-INH activity level of 32%, compared with 44% and 81% for those who received 3,000 and 6,000 IU doses, respectively.19 An analysis of the PK data further revealed that SC dosing provided a more even distribution of C1-INH activity levels across the body weight spectrum than IV dosing. A PK simulation showed that C1-INH(SC) administered as a weight-based dose of 60 IU/kg provided a markedly lower peak-to-trough ratio compared with the IV dose of 1,000 IU (1.3 versus 1.9) and critical C1-INH levels were better maintained after follow-up injections.20
In summary, C1-INH(IV) replacement therapy is effective and can provide a good level of protection if trough C1-INH levels can be consistently maintained above a critical threshold of 40% functional C1-INH.13 SC C1-INH administration as a volume-reduced formulation is feasible and provides a more consistent increase of trough C1-INH levels above the critical levels compared with IV C1-INH18,19 and thus is expected to be associated with an improved preventive effect.
COMPACT: Evidence for Subcutaneous C1-INH for Routine Prevention
Professor Bruce Zuraw
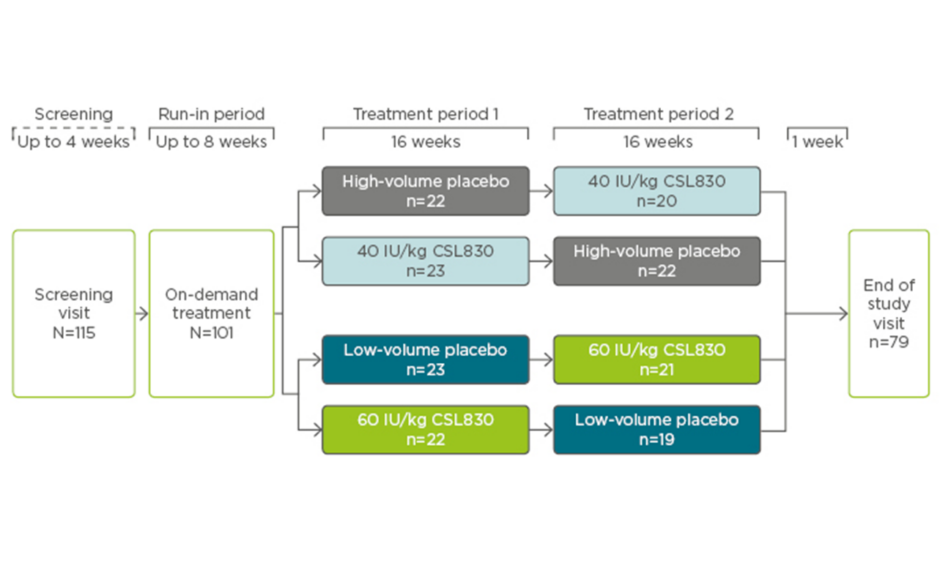
The international, prospective, multicentre, randomised, double-blind, placebo-controlled, dose-ranging, crossover Phase III COMPACT trial evaluated the efficacy and safety of self-administered formulation of a volume-reduced C1-INH concentrate, CSL830, in patients with Type I or II HAE. Patients were required to have had ≥4 attacks in a consecutive 2-month period within 3 months before screening and were included if they were aged ≥12 years, had HAE Type I or II confirmed by a central laboratory, and were stable on oral HAE prophylaxis. Those with a history of arterial/venous thrombosis that required anticoagulant therapy, were at risk of thrombosis, or were not adequately managed with on-demand treatment were excluded. On completion of the screening and run-in period, patients were randomised 1:1:1:1 to 40 IU/kg, 60 IU/kg, or low or high-volume placebo twice-weekly for 16 weeks (2 weeks to reach steady state, plus 14 weeks efficacy assessment). Following the first treatment period, patients were switched so that those assigned to placebo received active treatment for a further 16 weeks, or vice versa (Figure 1).21
Figure 1: COMPACT study design.
IU: international units.
Adapted from Longhurst et al.21
The primary efficacy endpoint was the time-normalised number of HAE attacks (investigator-reported); secondary efficacy endpoints included the percentage of patients who had a response (defined as ≥50% reduction versus placebo in number of attacks) and the time-normalised number of times rescue medication was used. Other endpoints included severity of attacks, safety and tolerability, and PK/pharmacodynamics analysis.21
In total, 90 patients were randomised to receive treatment in the first period, of which 91% (n=82) proceeded to the second treatment period (Figure 1). Patient demographics were relatively well-balanced between groups; the average age was 40 years, 67% were female, and 93% were Caucasian.21
Among patients who received CSL830, the rate of HAE attacks was lower compared with the rate among those who received placebo. Patients in the CSL830 lower dose group experienced 1.2 HAE attacks per month, compared with 3.6 in the placebo group (mean difference of -2.42 attacks per month; p<0.001). Patients in the CSL830 higher dose group experienced 0.5 HAE attacks per month, compared with 4.0 in the placebo group (mean difference of -3.51 attacks per month; p<0.001). The median reduction in the normalised number of attacks versus placebo was 89% and 95% with 40 IU/kg and 60 IU/kg of CSL830, respectively (Figure 2).21
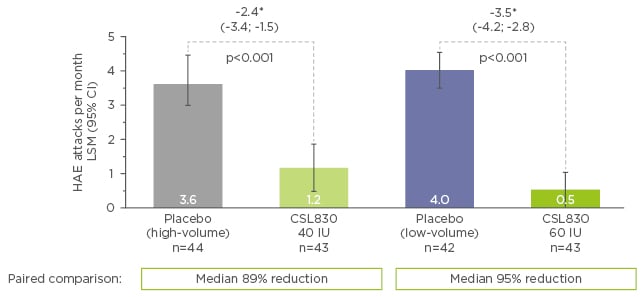
Figure 2: Time-normalised number of hereditary angioedema attacks per month.
*LSM (95% CI) estimate; error bars represent 95% confidence interval
CI: confidence interval; HAE: hereditary angioedema; IU: international units; LSM: least-squares mean.
Adapted from Longhurst et al.21
The mean normalised number of times rescue medication was used was reduced with both doses of CSL830 compared with placebo: -4.4 and -3.6 with 40 IU/kg and 60 IU/kg, respectively. The median reduction in the normalised use of rescue medication versus placebo was 89% for 40 IU and 100% for 60 IU. Of those treated with lower dose CSL830, 76% had a ≥50% reduction in the number of attacks, 67% had a ≥70% reduction, and 43% had a ≥90% reduction versus placebo. Of those treated with higher dose CSL830, 90% had a ≥50% reduction in the number of attacks, 83% had a ≥70% reduction, and 58% had a ≥90% reduction versus placebo. Overall, fewer patients in the pooled CSL830 group experienced severe HAE attacks compared with those in the placebo group (14% versus 71%, respectively). Most patients in the pooled CSL830 group experienced mild-to-moderate attacks (42%) compared with those in the placebo group (20%). In total, 39% of patients treated with CSL830 did not have any HAE attack versus 4% of placebo-treated patients (Figure 3).21
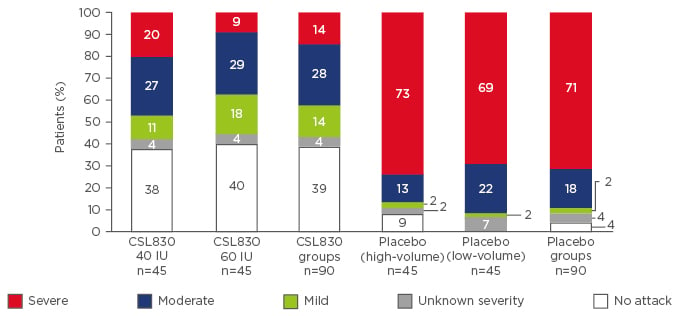
Figure 3: Attacks of hereditary angioedema according to the maximum severity.
IU: international units.
Adapted from Longhurst et al.21
Levels of functional C1-INH and C4 protein were measured at screening and throughout the study. At baseline, the levels of functional C1-INH activity and C4 protein were similar for all three groups and increased following randomisation in a dose-dependent manner until steady state was reached at Week 3. Patients treated with the 60 IU/kg dose had a mean increase in functional C1-INH activity that neared the lower limit of normal (approximately 70%) and those treated with the 40 IU/kg dose showed an intermediate increase to just below 50% functional activity. Levels of C4 protein were normalised for both dose groups; levels were maintained above the lower limit of normal with the 60 IU/kg dose and just below the lower limit of normal with the 40 IU/kg dose.21
Population-based exposure-response analysis revealed that there was an inverse relationship between the predicted functional C1-INH activity and the time of an attack, which suggests that if C1-INH activity is maintained close to the lower level of normal, the risk of a patient having an HAE attack approaches zero.21
Patient-reported quality of life (QoL) outcomes (European QoL-5 dimensions questionnaire; treatment satisfaction questionnaire for medication, hospital anxiety, and depression scale; work productivity and activity impairment questionnaire) were measured at screening and various times during the two treatment phases. Improvements were seen for all QoL outcomes versus placebo for both lower and higher dose CSL830, and were statistically significant for the visual analogue scale, treatment satisfaction (effectiveness and overall satisfaction), anxiety, and presenteeism.22
Most reported AE were injection-site reactions, which occurred in 31% of patients treated with CSL830 (28% and 35% of patients in the 40 and 60 IU/kg groups, respectively) and 24% of placebo-treated patients. Of the injection-site reactions, 95% in the CLS830 and placebo groups were of mild severity and ≥83% resolved within 1 day of onset. Other reported AE were not deemed to be related to the study drug and included nasopharyngitis, upper respiratory tract infection, hypersensitivity (pruritus, rash, and urticarial), dizziness, fatigue, and back pain.21
Overall, both doses of CSL830 significantly lowered the rate of HAE attacks compared with placebo and were associated with improvements in QoL measures. The higher (60 IU/kg) dose was more effective at reducing the attack rate and medication use compared with the lower (40 IU/kg) dose, and both were more effective than placebo. AE were mostly injection-site reactions, generally mild and transient in nature, and occurred in similar proportions of patients in the active treatment groups and placebo. The two treatment periods were not designed to assess the long-term effects of SC CSL830; however, an open-label extension trial23 is currently ongoing to assess safety and explore whether dose adjustments can further improve treatment response.
Question and Answer Session
Q: Should we personalise doses in clinical practice based on functional C1-INH levels?
A: Prof Craig replied that there is currently insufficient evidence to suggest that this is needed or useful, although it is an interesting research topic that deserves further exploration.
Q: Q: Could we start at a low dose and increase it based on patient response?
A: Prof Magerl replied that there is variation from patient to patient in frequency of symptoms, frequency of relapse, the interval between doses, and the appearance of symptoms, so future efforts should identify patient characteristics that could guide physicians and help to individualise therapy.
Prof Zuraw added that the future target is not to start at a given dose and adjust it, but rather to be able to predict which drug for which patient is best; this is something that may become a reality as the repertoire of drugs increases.
Q: When starting a patient on SC C1-INH, would you first administer an IV bolus or would you start on SC?
A: Prof Magerl replied that after 2–3 injections, C1-INH plasma levels reach what is considered an ‘effective’ level and therefore no initial bolus should be needed. In his view, C1-INH(SC) can be administered from the outset as prophylaxis.