Chairperson: Susanne Lau1
Speakers: Stephanie Dramburg,1 Marek Jutel,2,3 Petra Zieglmayer4
1. Department of Pediatrics, Division of Respiratory Medicine, Immunology and Critical Care Medicine, Charité Universitätsmedizin, Berlin, Germany
2. ALL-MED Medical Research Institute, Wroclaw, Poland
3. Department of Clinical Immunology, Wroclaw Medical University, Poland
4. Competence Center for Allergology and Immunology, Karl Landsteiner University, Krems, Austria
Disclosure: Lau has received lecture fees/participated in the advisory boards for ALK, Allergopharma, DBV Technologies, LETI Pharma, and Sanofi-Aventis; and was on the advisory board for Leo Pharma. Dramburg has received lecture fees from Allergopharma, Allergy Therapeutics, Bencard Allergie, and OMRON Healthcare; and has been a consultant for Allergy Therapeutics, Bencard Allergie, LETI Pharma, and OMRON Healthcare. Jutel has been a clinical trial investigator for Allergopharma, ALK, Allergy Therapeutics, Celltrion, Chiesi Farmaceutici, Genentech, GlaxoSmithKline, HAL Allergy Group, Janssen, LETI Pharma, Novartis, Roche, Sanofi Regeneron, Shire, Stallergenes, Takeda, and Teva Pharmaceuticals; was on the speakers’ bureau/received honoraria from ALK, Allergopharma, Chiesi, GlaxoSmithKline, HAL Allergy Group, Novartis, and Stallergenes; and was a consultant/on the advisory board for ALK, Allergopharma, Chiesi, HAL Allergy Group, and Stallergenes. Zieglmayer has received lecture fees from ALK-Abelló, Allergopharma, Allergy Therapeutics, Bencard Allergie, HAL Allergy Group, LETI Pharma, MadX, Meda Pharmaceuticals, Merck & Co., Novartis, Stallergenes Greer, and Thermo Fisher Scientific; and has received scientific and educational grants from ALK-Abelló, Allergopharma, Allergy Therapeutics, Biomay, Calistoga Pharmaceuticals, GlaxoSmithKline, HAL Allergy Group, Marinomed, MSD, Ono Pharamceutical, Oxagen Pharma, RespiVert, Stallergenes Greer, and VentiRx Pharmaceuticals.
Acknowledgements: Medical writing assistance was provided by Brigitte Scott, MarYas Editorial Services, Cowlinge, UK.
Disclaimer: The views and opinions expressed are those of the speakers and not necessarily of Allergopharma GmbH & Co. KG.
Support: The publication of this article was funded by Allergopharma GmbH & Co. KG.
Citation: EMJ Allergy Immunol. 2022;7[1]:22-32. DOI/10.33590/emjallergyimmunol/10192957. https://doi.org/10.33590/emjallergyimmunol/10192957.
Meeting Summary
This symposium took place during the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress, held in Prague, Czechia, in July 2022. Stephanie Dramburg, Medical Doctor at Charité Universitätsmedizin, Berlin, Germany, explained that the traditional diagnostic work-up for allergic rhinoconjunctivitis (AR) comprises retrospective clinical history and allergen exposure assessment, extract-based diagnostics, component-resolved diagnostics (CRD), which enables markers of genuine sensitisation to be distinguished from markers of cross-reactivity, and confirmation of clinical relevance. She highlighted that molecular IgE assessment supports the diagnostic work-up and personalised risk assessment in complex cases and that confirmation of clinical relevance of IgE results is key. Furthermore, broadening of a serum IgE response is indicative of ‘molecular spreading’. Dramburg considered that digital technologies have the potential to enhance medical decisions at the point-of-care via targeted patient information, guideline- and evidence-based clinical knowledge, and prospectively collected patient- and sensor-generated data. Marek Jutel, Medical Professor at Wroclaw Medical University, Poland, and the ALL-MED Medical Research Institute, Wroclaw, Poland, described that patients with allergies show different clinical pictures due to differing sensitisation profiles assessed at the molecular level. He noted that patients with allergies react differently to different allergen doses and allergen immunotherapy (AIT) preparations, and minor/intermediate allergens are necessary, in addition to major allergens, for optimal clinical efficacy. Jutel described that allergens that are decisive for AIT efficacy are defined in grass pollen but are not yet determined for other allergen sources such as birch and house dust mite (HDM). Petra Zieglmayer, Medical Professor at Karl Landsteiner University, Krems, Austria, and Head of Vienna Challenge Chamber, Austria, discussed that patients with allergies show complex molecular sensitisation profiles and that extract preparations from different manufacturers vary in terms of allergen composition, with major and intermediate allergens not always detectable. She clarified that optimal efficacy of AIT may only be expected from preparations containing all relevant allergen components in sufficient amounts. Zieglmayer proposed that the target should be to find a match between the patient molecular sensitisation profile and the allergen preparation and that this can be achieved.Patients’ Phenotype Assessment: A Chance for Digital Technologies?
Stephanie Dramburg
Dramburg explained that the traditional diagnostic work-up for AR comprises retrospective clinical history and allergen exposure assessment, extract-based diagnostics, CRD, and confirmation of clinical relevance. Focusing on retrospective clinical history, Dramburg pointed out that patients already use digital technology in the form of calendars on their phones to help them remember and record their symptoms (date, location, and pollination season).
In regard to retrospective exposure assessment, Dramburg described that symptom seasonality is associated with allergen exposure and there is little overlap between the different pollen seasons in Germany so far.1 Therefore, the agents eliciting symptoms can generally be identified according to when symptoms occur. However, Dramburg highlighted that pollination periods are expanding and that countries in northern and central Europe need to be prepared to see patterns already found in southern Europe (e.g., Italy), with overlapping pollen seasons that complicate the identification of the allergens eliciting symptoms.2
This overlapping pollination pattern is also reflected in the patient sensitisation profiles in southern Europe. In a study by the Italian Paediatric Allergy Network (I-PAN), in which 1,360 children aged 4–18 years were recruited, over 80% of participants had a positive skin prick test (SPT) result to ≥3 pollens and almost 50% reacted to ≥6 pollens.3 Dramburg noted that it would be difficult to prescribe immunotherapy in such cases, as ascertaining the correct allergen to target would be challenging, and this is where CRD is important: the use of recombinant or highly purified native allergenic molecules enables markers of genuine sensitisation to be distinguished from markers of cross-reactivity.
Population data from the German Multicentre Allergy Study birth cohort4 of 820 children (of 1,314 recruited) indicated that the prevalence of IgE sensitisation to grass pollen starts to rise before the onset of clinical symptoms, mainly for major allergens such as Phleum pratense (Phl p) 1 and 5, but also over time for minor and cross-reactive molecules, including Phl p 11 and 12. Dramburg questioned what this population data means for individual patients and discussed the case of a paediatric patient as a clinical example.5 The first blood sample from this patient was taken at age 3 years, when the patient had no allergy symptoms but already had specific IgE (sIgE) to Phl p 1. Onset of symptoms was at age 6 years, when there was evidence of a broadening of sIgE response to Phl p 1, 2, and 4; so-called ‘molecular spreading’. At age 10 years the patient was broadly sensitised, including to cross-reactive molecules, and was considered to potentially react to other botanic sources.
Further population data from the German Multicentre Allergy Study birth cohort4 showed that the molecular spreading phenomenon was also evident for HDM allergenic molecules, with Der p 1, 2, and 23 considered to be ’initiator molecules’ as they appear early in the sensitisation process.6
The transition of a patient along their sensitisation journey is one-way, towards a broader sensitisation spectrum, with no patients reverting to a narrower immune response.6 Dramburg disclosed that molecular IgE diagnostics help give clinicians a better idea of where the patient is in their sensitisation journey, i.e., are they still mono-sensitised, do they recognise several allergens or allergenic proteins (oligo-/poly-sensitisation), is there any response to marker molecules (e.g., Dermatophagoides pteronyssinus [Der p] 23 as an indicator of asthma risk), or are there markers of cross-reactivity?
The use of CRD has a significant impact on the selection of the AIT formula. In an I-PAN study of 651 children with moderate-to-severe AR, clinicians initially based diagnosis and therapeutic decisions on SPT results; however, when CRD data were available, the decision about the AIT formula was changed in up to 18% of cases.7
Dramburg then discussed confirmation of the clinical relevance of molecular test results, which for AR involves nasal8 or conjunctival9 allergen provocation tests. These tests can be challenging and time-consuming in patients who are poly-sensitised. In such cases, symptom recording by patients using digital technology, such as an app that combines a symptom diary (electronic [e]-diary) with pollen and weather data, may help clinicians to better understand the phenotype of their patients.
Two patients who were sensitised to multiple seasonal allergens (as shown by SPT results) with overlapping pollination periods were discussed. In these cases, the use of molecular diagnostics did not help to narrow down the likely allergens eliciting the symptoms.10 Therefore, the patients were asked to monitor their symptoms themselves and pollen exposure was monitored separately. The rhinoconjunctivitis total symptom score for the two patients correlated perfectly with exposure to a specific allergen (olive pollen for one patient and grass pollen for the other).10 According to Dramburg, the data from patient-recorded symptom e-diaries are helpful for clinicians when deciding which AIT to use first, or for defining which is the clinically relevant agent for the patient.
Dramburg proposed a clinical decision support system (CDSS) could be used to assist clinicians in their day-to-day practice.11 A digital system algorithm takes into account factors such as clinical history and allergen exposure assessment, extract-based diagnostics, CRD, and evidence-based guidelines, as well as treatment settings and local adaption of the support system according to the clinician’s personal experience.11 The patient is repeatedly re-evaluated according to the algorithm to monitor success and ensure the timeliness of any treatment adaptions.
There have been different approaches for CDSS algorithms in allergic rhinitis, including an expert opinion-based decision algorithm for symptomatic treatment (MASK e-CDSS) from the Allergic Rhinitis and its Impact on Asthma (ARIA) consortium,12 and a diagnostic algorithm based on CRD and e-diaries (@IT2020-CDSS) that was evaluated in a pilot study in Italy by Arasi et al.13
As part of the @IT.2020 multicentre study,14,15 815 patients aged 10–60 years with seasonal allergic rhinitis were recruited in seven countries in southern Europe. Patients completed clinical questionnaires and underwent SPTs and sIgE testing. A symptom diary app (AllergyMonitor, Technology & Project Software [TPS] Production, Rome, Italy) was installed on their phones.14 Patients were specifically asked to monitor their symptoms according to their suspected relevant allergen season (based on SPT results and clinical history) rather than throughout the year, and the resulting information was communicated to the patients and provided to clinicians to see if it improved decision-making.14
Dramburg emphasised that the focus of digital technology should ideally support the patient–clinician relationship as part of a blended care approach, where technology assists but does not replace the clinician. In the @IT.2020 multicentre study,14,15 for example, clinicians prescribed symptom monitoring as diagnostically necessary. Patient adherence to symptom e-diary recording in this blended care setting was very high, with around 80% adherence during a period of over 70 days.16
Dramburg summarised the three steps in the CDSS as clinical history (symptom seasonality) and SPT and/or sIgE; CRD; and e-diary pollen exposure, with the aim to provide more precise prescription of AIT based on good data. In the pilot study,13 a workshop with allergy specialists and general practitioners showed that knowledge gleaned from each of the three steps of the CDSS was associated with improved diagnostic performance. This indicated the theoretical potential of the CDSS for supporting clinicians in their diagnostic decision-making (Figure 1).
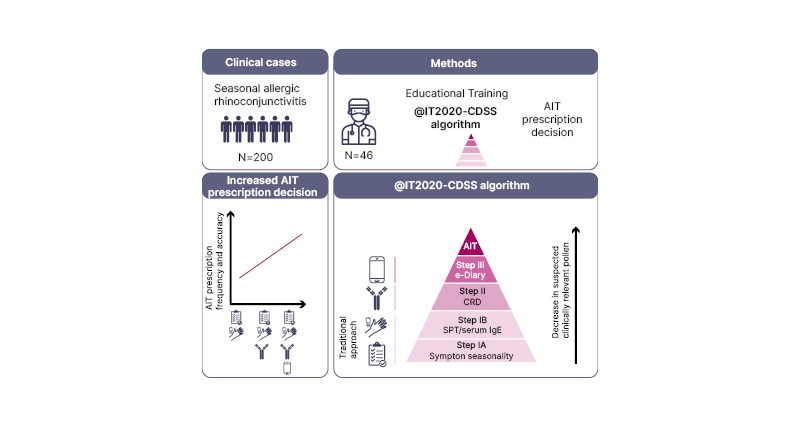
Figure 1: Three steps in the Clinical Decision Support System.13 AIT: allergen immunotherapy; CRD: component-resolved diagnostics; e-Diary: electronic diary; SPT: skin prick test.
The clinicians’ opinions of the usability of the CDSS were then explored. The allergy specialists and general practitioners considered that the accuracy of AIT prescription can be improved with a CDSS, the proposed algorithm makes sense, and it enhances traditional diagnostics. Notably, they also questioned the reliability of clinical history that is retrospectively assessed.
Dramburg concluded that molecular IgE assessment supports the diagnostic work-up and personalised risk assessment in complex cases and that confirmation of clinical relevance of IgE results is key. Software tools and mobile solutions have the potential to enhance medical decisions at the point-of-care via targeted patient information, guideline- and evidence-based clinical knowledge, and prospectively collected patient- and sensor-generated data. Dramburg emphasised that patients or their caregivers are not recommended to record symptoms until they have clearly occurred to prevent patients being considered as “sicker than they are.”
Matching Molecular Profiles and Clinical Outcomes
Marek Jutel
Jutel reported that patients show different sensitisation profiles, which can be origin-dependent. This is illustrated in a study by Muddaluru et al.17 conducted in Canada, Europe, South Africa, and the USA, which showed different IgE responses to HDM allergens based on geographical location. Differences in molecular profile are also seen in patients according to clinical diagnosis. Resch et al.18 showed that the IgE profiles to individual HDM allergens of children with or without asthma with HDM allergy differed in terms of IgE binding prevalence and the number of allergens recognised. Interestingly, the patients with asthma were predominantly sensitised to a larger number of allergens in the allergen source than the patients without asthma.18
Jutel noted that there are several biomarkers of humoral and cellular immune response that can be assessed during the course of AIT, including sIgE, sIgG4, interleukin-4 (IL-4)+ cells, regulatory T cell response, and eosinophil count.19 Jutel referred to a study by Shamji et al.20 that showed the time course and dose-dependency of clinical outcome, allergen-specific IgG4 antibody levels (measured by enzyme-linked immunosorbent assay) and serum inhibitory activity (measured using the IgE-facilitated allergen binding assay [IgE-FAB], which assesses functional IgG4) during subcutaneous grass pollen AIT. Levels of functional IgG4 correlated closely with clinical response to AIT.20
Jutel explained that different allergen extracts induce dissimilar grass pollen allergen-specific IgG responses in an experimental model in rabbits,21 because of the variations in the composition and number of allergens in these preparations. Following on from this preclinical observation, Jutel posed the question: Why do patients with different molecular profiles respond differently to different AIT preparations? In addressing this question, he highlighted the disparity between traditional allergen extracts and molecular extracts. Traditional extracts involve extraction and purification (e.g., of a grass sample) and then the composition and strength (amount of allergen) of the extract is evaluated. In contrast, the exact qualitative and quantitative composition of molecular extracts (recombinant preparations) is established a priori by the manufacturer and is known to the clinician and the regulatory agency.22
In consideration of a further question about how patients with different molecular profiles react to a molecular extract, Jutel reiterated that there are major grass pollen allergens such as Phl p 1 and 5, to which the majority of patients are sensitised; intermediate allergens, including Phl p 2, 4, and 6, to which some patients are sensitised; and minor allergens, like Phl p 3, 7, 11, 12, and 13, to which few patients are sensitised.
The link between patients’ molecular profiles, a molecular extract and clinical outcomes was assessed post-hoc in a double-blinded, placebo-controlled study (AL0704rP; EudraCT 2007-003208-37; [Allergopharma GmbH & Co. KG, unpublished data]), which involved a recombinant equimolar preparation of Phl p 1, 2, 5a, 5b, and 6. The clinical outcome after AIT was assessed in the context of the sensitisation profiles of the patients before the initiation of AIT treatment. The primary endpoint was rhinoconjunctivitis symptom medication score (RC-SMS). Jutel pointed out that, importantly, there was no Phl p 4 allergen in this preparation.
According to Jutel, numerous questions arose while analysing the data from study AL0704rP (Allergopharma GmbH & Co. KG, unpublished data), including whether the composition of the allergen cocktail was optimal (or were any relevant allergens missing), whether only major allergens are necessary for optimal efficacy, or does such a strong cocktail of allergens induce new sensitisations in patients, and whether there are any differences in efficacy in patients with broad (poly-) versus mono-/oligo-sensitisation (Nandy, unpublished data).
Jutel explored whether the sensitisation profile of the patient before AIT could be regarded as a prognostic biomarker for treatment efficacy. Analysis of the molecular profile of participants who had ≥70% improvement in RC-SMS after 2 years of active treatment (120 μg allergen, which is six-times higher than the 20 μg considered sufficient to induce a response) showed that sensitisation to Phl p 1 and 5 is important, with sensitisation to at least one of these allergens necessary for AIT efficacy (Allergopharma GmbH & Co. KG, unpublished data). There was a low level of sensitisation to minor and intermediate allergens (Phl p 7, 11, 12, and 13 [Allergopharma GmbH &Co. KG, unpublished data]); however, Jutel clarified that these allergens are still important and cannot be disregarded, i.e., it is not only major allergens that are necessary for optimal efficacy. Supportive data on the importance of Phl p 1 and 5 as predictive biomarkers for treatment efficacy were provided by a study of sublingual immunotherapy, where patients with low pre-treatment sIgE to Phl p 1 or 5 presented no clinical benefit in the first pollen treatment season.23
Those participants who had ≥40% deterioration in RC-SMS after 2 years of active treatment were sensitised at inclusion to Phl p 4, which was not included in the study preparation (Allergopharma GmbH & Co. KG, unpublished data). Jutel stated that the composition of the allergen cocktail was not optimal and Phl p 4 is essential in an AIT preparation for assessment of theprojected treatment efficacy.
Two-thirds of patients (10 out of 15) who were not sensitised to Phl p 4 at inclusion showed improvement in RC-SMS after 2 years of active treatment (Allergopharma GmbH & Co. KG, unpublished data). The remaining five patients who showed deterioration in RC-SMS were sensitised to Phl p 1 but not to Phl p 5 (Allergopharma GmbH & Co. KG, unpublished data), which indicates that sensitisation to Phl p 5 before AIT may be of higher importance than that to Phl p 1 for treatment efficacy.
Jutel then considered whether the recombinant allergen cocktail induces new sensitisations because of a mismatch between the composition of the preparation and the patient’s sensitisation profile before treatment.24 A study of the sensitisation pattern of 176 children showed that there was a 100% match to cocktail composition in only 4% of patients, and 67% of patients were sensitised to either more allergens than were in the cocktail, or some or no allergens that were in this preparation.25 Although this may cause concern in terms of raising new sensitisations when using AIT preparations containing more allergens than the patient is sensitised to, the study confirmed that this is not the case by showing that there were no significant differences in new sensitisations between active- and placebo-treated patients (Table 1 [Allergopharma GmbH & Co. KG, unpublished data]). Jutel also clarified that no differences in response to allergen preparations have been seen in patients who have poly- versus mono-/oligo-sensitisation (Nandy, unpublished data).
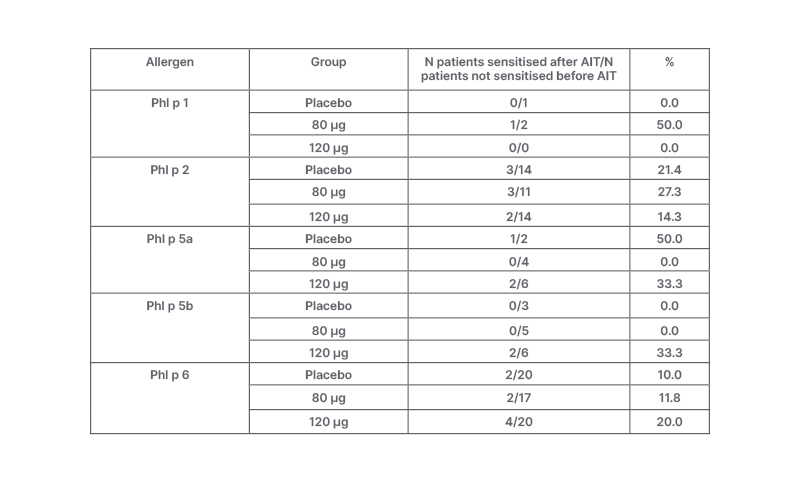
Table 1: New sensitisations in patients not sensitised against the respective allergen before treatment.
Allergopharma GmbH & Co. KG, unpublished data. AIT: allergen immunotherapy; Phl p: Phleum pratense.
Jutel concluded that patients with allergy show different clinical pictures probably due to their different sensitisation profiles assessed at the molecular level. Patients with allergy react differently to different allergen doses and AIT preparations, and minor/intermediate allergens are necessary, in addition to major allergens, for optimal clinical efficacy. There are several unmet needs in AIT. Allergens that are decisive for AIT efficacy are defined in grass pollen to be Phl p 1, 4, and 5; however, those for other allergen sources (birch and HDM) are yet to be determined. Jutel assumed that it may be possible to design a universal allergen-based preparation for grass and birch pollen or cat allergy, but not for sensitisation to complex allergen sources (e.g., mites, moulds).
Optimal Allergen Compositions: A Benefit for the Patient?
Petra Zieglmayer
Zieglmayer emphasised that commercial allergen extract preparations used for diagnostics and treatment are derived from natural materials and their molecular composition varies regarding allergen content and concentration.26 Also, as explained by Jutel using grasses as an example, it is important to consider minor and intermediate allergens as well as major allergens for these extracts. The importance of Group 5 allergens for the success of AIT is recognised;27 however, that of Group 1 allergens is still unclear, and these allergens are underrepresented in some commercial extracts because of problems with extractability, degradation in stored extracts, and diverse immunogenicity.27 According to Zieglmayer, the allergenicity of Group 5 allergens in terms of induction of a protective IgG4 response is far higher than it is for Group 1 allergens, depending on the allergen content and formulation of the preparation. Therefore, there is a need to optimise the content of preparations and their adaptability.
Zieglmayer noted that commercially available HDM extracts are also heterogenous, with the composition of these extracts depending on the source of material used (e.g., whole mite cultures, including faeces, versus a purified mite body source material with no faeces).28 She indicated that extracts that differ in terms of major and intermediate mite allergen content may produce different benefits for patients when used as AIT and questioned how this can be managed.
Focusing on the production process, Zieglmayer described how the timing and method of extraction impacts on allergen content. For example, extracts from different harvests of birch pollen material have been shown to have naturally diverse composition, and process steps like the extraction time impact on the allergen content.29 Therefore, there is a clear batch-to-batch variability between extracts derived from natural materials that Zieglmayer suggested needs to be managed accordingly, through adapting production processes to optimise the composition of the preparations. Another quality assurance measure is to fully characterise the composition of allergen preparations using mass spectrometry.30 This provides further information about the match between sensitisation profile of the patient and composition of the preparation required for treatment.
Zieglmayer presented the allergen components in different grass pollen preparations (Table 2) and emphasised that it is possible to have all relevant grass allergens in these preparations, whether they are native or allergoid, or subcutaneous or sublingual, but this is not a given.31 As shown by the crosses in Table 2, some important allergens may be missing, which may impact on the efficacy of immunotherapy.31
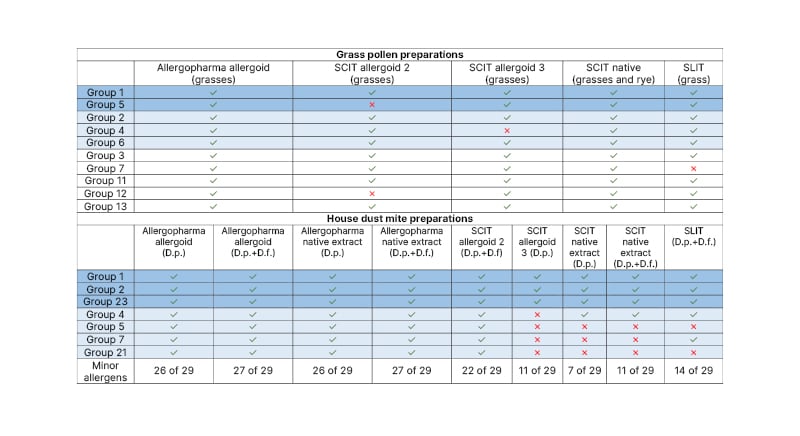
Table 2: Allergen components in different grass pollen preparations and different house dust mite preparations.31
Dark blue: major allergen; light blue: intermediate allergen; white: minor allergen.
D.f.: Dermatophagoides farinae; D.p.: Dermatophagoides pteronyssinus; SCIT: subcutaneous immunotherapy; SLIT: sublingual immunotherapy.
Analysis of the complete allergen spectrum of HDM preparations showed that it is possible to include all relevant HDM allergens whether it is a natural, unmodified, or a modified (allergoid) preparation,32-34 which indicates that the composition is not dependent on the formulation. However, Zieglmayer stressed that clinicians should be aware that HDM preparations from different manufacturers vary (Table 2), and some may lack relevant allergens to which patients are sensitised.31
As part of a discussion on the impact of treatment composition on the efficacy of AIT, Zieglmayer pointed out that there are grass pollen species that are more relevant for northern than southern Europe and vice versa,35 and she questioned whether these species are interchangeable or whether it is sufficient to use one representative species for all patients, irrespective of their location. She remarked that there is a close phylogenetic relationship between the sweet grass species in northern Europe.36 However, a grass species from southern Europe (Cynodon dactylon or Bermuda grass) shows low cross-reactivity with the northern European sweet grasses because it does not contain Group 5 allergens and has different Group 1 epitopes.37
Zieglmayer highlighted that the immune system can differentiate between grass pollen species, and that Timothy grass (Phl p; a species that is always included in testing) is not representative of all grass species in terms of allergen composition.38 How this impacts on management of patients regarding AIT was investigated in a small study in which patients from northern and southern Europe received one- or five-grass pollen sublingual immunotherapy tablets.39 Inhibition of IgE binding to pollen allergens from 12 grasses was significantly stronger with the five-grass than with the one-grass pollen tablet (p<0.0001), with five out of six patients managing inhibition, regardless of whether patients were considered as a whole or by geographical area.39 The one-grass pollen tablet may be sufficient for most patients to manage the inhibition of a standard 12-grass pollen mix but the remaining patients (one out of six) may not be covered adequately having received just one representative grass pollen rather than a mixture of grass pollen species.
Referring again to mites, Zieglmayer stated that molecular HDM sensitisation profiles are complex,40 so there is likely no one-size-fits-all solution. Patients who were treated subcutaneously with a standard mite preparation for 1 year in a placebo-controlled, prospective study were evaluated post-hoc for their sensitisation profiles against 12 Der p allergen components at inclusion (three major allergens [Der p 1, 2, and 23], four intermediate allergens [Der p 4, 5, 7, and 21], and five minor allergens [Der p 10, 11, 14, 15, and 18]).41 According to Zieglmayer, the results were surprising because patients developed an immune response (HDM-specific IgG) against Group 1 and 2 major allergens, whereas the level of IgG response was not different from that in the placebo group for Group 23 or the intermediate allergens, Der p 5, 7, and 21 or minor allergens.41 Therefore, only the former two major allergens were considered to be present in sufficient amounts in the preparation. This was clinically relevant as only patients who were oligo-sensitised to Group 1 and 2 HDM allergens showed clinical benefit after 1 year of treatment.41 In contrast, standard patients with a complex profile showed no such clinical benefit after 1 year of treatment. Clinical benefit of subcutaneous immunotherapy, therefore, depends on a fit between the patient’s molecular sensitisation profile and the extract molecular profile. One option to achieve this fit, therefore, is to treat patients with modern preparations that contain all relevant allergens.
Notably, a modern HDM tablet preparation with a fractioned composition has shown significant and persisting clinical improvement, with the marketed dose associated with a significant reduction in nasal, ocular, and asthma symptoms within only 8 weeks of treatment, and the improvement maintained at 1-year of follow-up.42,43 These results indicate a match between the sensitisation profile of the patient and the allergen composition of the preparation.
Zieglmayer stated that there is a general need to evaluate the relevance of formulations per se and how dose-dependent effects are but ‘the composition counts’.
Zieglmayer concluded that patients with allergy show complex molecular sensitisation profiles and that extract preparations from different manufacturers vary in terms of allergen composition, with major and intermediate allergens not always detectable even by mass spectrometry. Optimal efficacy of AIT may only be expected from preparations containing all relevant allergen components in sufficient amounts. The target should be to find a match between the patient molecular sensitisation profile and the allergen preparation, and this can be achieved.
Concluding Remarks from the Chair
Susanne Lau
Susanne Lau, Professor at Charité Universitätsmedizin, Berlin, Germany, summarised that patients show different patterns of sensitisation, particularly for grass pollen and HDM allergens, and several allergens play a role. She also referred to the important differences in allergen extract preparations. Lau suggested that these considerations and the data presented may create confusion for prescribers of AIT in terms of whether CRD should be performed for all patients with grass pollen allergy, and that, for now, it is best to choose an extract that includes all relevant allergens. Other considerations she raised included how could digital technology help clinicians to be more precise about the significance of sensitisations, how strongly should clinicians encourage patients to record data, how much data should they record, and in which way will clinicians use the patient-recorded data. Questions such as these will direct future research in this evolving area to enable optimal patient care.






