Abstract
Since its introduction, the success of percutaneous transluminal coronary angioplasty (PTCA) has been jeopardised by recoil, neointima proliferation, and luminal renarrowing; however, the benefit of positive remodelling has not gained widespread attention. While vessels will remodel positively up to a certain stage in the development of atherosclerosis, the therapeutic application of this process remains low. The prevention of vessel shrinkage during the healing process, which represents the predominant mechanism of restenosis after PTCA, is a prerequisite of long-term success in PTCA. The antiproliferative drugs that are currently used mainly on stents are known to be capable of this. Primary clinical studies have reported that positive remodelling leads to beneficial effects in coronary and peripheral angioplasty if no foreign body is inserted, and a paradigm change in percutaneous coronary intervention towards far fewer implants is within reach.
INTRODUCTION
The success rate of percutaneous peripheral and coronary angioplasty has been hindered by restenosis since its introduction by Dotter and Judkins,1 and Grüntzig.2 Initially, the main obstacles faced were elastic recoil and neointima proliferation, which contributed to luminal renarrowing. Bare-metal stenting was the initial solution; however, it was revealed to have only a marginal benefit due to the exaggerated neointima hyperplasia initiated by the insertion of a foreign body.3 Drug-eluting stents (ES) have therefore been implemented to counteract the foreign body reaction, and this has proved especially successful in reducing reinterventions.4 Drug-ES have therefore evolved as the current default solution.
There has not been as much research on the external size and the consecutive internal lumen changes of blood vessels, both during the development of atherosclerosis and after angioplasty. The stenting of most lesions restricts the ability of blood vessels to expand as part of the healing process, which is now sometimes referred to as vascular restoration. Both the positive remodelling effects in the natural disease process, as well as the antiproliferative drug-induced vessel enlargement, are greatly counterbalanced by neointima proliferation and a rigid stent-fortified vessel wall. Even with the use of bioresorbable scaffolds, the net effect, in spite of all methodological measurement flaws in the first year, is negative remodelling (luminal narrowing), while it is only later that part of the early narrowing is counteracted by vessel enlargement.5 Other foreign body-associated problems, such as an enhanced thrombosis rate, remain.6 Thus, by stenting or scaffolding most lesions, the ability of blood vessels to expand as part of the healing process or induction of vessel size increase by antiproliferative agents has not been recognised as a therapeutic option.
VESSEL REMODELLING
Though under-recognised, positive vessel remodelling is a commonly observed phenomenon in vascular medicine. Most cardiologists are familiar with remodelling of the internal mammary artery after connecting it to the left anterior descending coronary artery: it grows to meet the blood flow demand. Similarly, arteriovenous shunts for dialysis patients will grow after the arteriovenous connection is established. In their seminal work, Glagov et al.7 describe how vessels will enlarge (positive remodelling) during the development of atherosclerosis, as long as plaque material does not comprise more than 40% of the cross-sectional vessel area. In this process, compensatory positive remodelling will prevent luminal narrowing until the later stages of the atherosclerotic process. In addition, a dilative form of atherosclerosis occurs in some patients.
Unwanted positive vascular remodelling occurs after application of drug-ES and can cause secondary stent malapposition if the neointimal proliferation, caused by the foreign body reaction, does not compensate the luminal change.8 This is thought to be one of the leading causes of late and very late stent thrombosis,9-11 and is also thought to differ in magnitude between different antiproliferative drugs, excipients, and stent designs.12,13 Paclitaxel-ES and sirolimus-ES seem to cause more vascular remodelling than everolimus-ES and zatarolimus-ES; the pharmacologic reason for this difference is not entirely clear. The drug effect leading to positive remodelling in this setting is counteracted by neointimal hyperplasia covering the stent mashes. Without a foreign body reaction, the expected net effect would be luminal enlargement.
More than 20 years ago, before the use of stents was widespread, Currier and Faxon14 studied restenosis after percutaneous transluminal coronary angioplasty (PTCA), questioning whether therapy was aimed at the wrong target for addressing predominantly neointimal hyperplasia. He pointed to a PTCA-induced decrease in vessel size, measured as the area comprised by the internal elastic membrane, and suggested a therapy directed at arterial remodelling, which was not available at this time.
After plain old balloon angioplasty, most vessels heal with negative vessel remodelling. This means that the healing process causes an overall shrinkage of the cross-sectional area in the treated vessel segment, leading to luminal narrowing.15,16 This vessel shrinkage is the predominant mechanism of restenosis after PTCA, while neointimal proliferation is the predominant mechanism after stenting, proven both experimentally and clinically through intravascular ultrasound.17-19
THERAPEUTIC UNDERESTIMATION OF VESSEL REMODELLING
With regard to the aforementioned, it is not surprising that the potential of positive remodelling to increase the lumen of atherosclerotic vessels as an interventional therapy has been underestimated. Paclitaxel is the best-evaluated drug in this context. Once absorbed, its hydrophilic nature allows the drug to stay in the arterial vascular wall for a prolonged period of time. Its inhibitory action on smooth muscle cell proliferation is caused by modulation of the microtubule formation and by upregulation of proapoptotic factors.20,21 The mechanism of positive remodelling is thought to be the apoptosis of smooth muscle cells. Thus, the pathophysiology of spontaneous increased vessel size in the early stages of atherosclerosis is mimicked by the pharmacological effects induced by paclitaxel.
The effect of paclitaxel was found to be clinically unfavourable when, in the TAXUS II trial,22 it was reported that it led to increased vessel size in stented areas. It also induces the increase of carotid vessel size after balloon injury if applied locally,23 and the effect on vessel size is far greater than the effect on the reduction of neointimal proliferation.24 Locally applied paclitaxel (applied during contrast injection,25 by local application on a balloon using an appropriate excipient,26 or by injection of paclitaxel into the pericardial sack27) leads to sufficient vascular tissue concentration to induce a sizable increase in vessel diameter. Unlike siromilus, paclitaxel is thereby able to induce apoptosis of smooth muscle cells. This leads to a decrease in medial and intimal smooth muscle cells, and in collagen content.21 While this induces a theoretical risk of coronary artery aneurysm formation, the study of a large number of patients has not found an excess rate of coronary artery aneurysms after drug-coated balloon (DCB) angioplasty.28
The Effect of Alternative Drugs
Several limus-based drug balloons are currently in clinical development and some have even earned the Conformité Européenne safety mark. So far, these have only been tested in in-stent restenosis settings; however, a clinical proof of positive remodelling with these compounds is still pending. While neointimal thickening seems to be better suppressed by sirolimus than by paclitaxel,29 and sirolimus still has more effect on the vessel size than zatarolimus does, animal data suggest that sirolimus has a somewhat weaker effect on vessel size increase than paclitaxel.29-31
Preliminary Clinical Evidence
Primary clinical studies have provided evidence that positive remodelling leads to beneficial effects in coronary32,33 and peripheral34 angioplasty if no foreign body is implanted. A sizable percentage of cases exhibited a luminal increase of the coronary arteries from end of procedure to 4 months after, obviating the need to end angioplasty without residual stenosis (Figure 1). The average luminal diameters after DCB angioplasty either increased or showed no luminal renarrowing in >80% of treated lesions.32,33 Since the initial report using quantitative angiography, these data have been confirmed by intravascular ultrasound and optical coherence tomography in various trials and by various groups.35-37 It has been found that even side-branch ostia benefit from main vessel drug application. Using optical coherence tomography, Her et al.38 described an increase of the ostial side branch area from 1.0–1.4 mm2 (40%) without even touching the side branch during intervention, with insightful optical coherence tomography images available in the publication.38
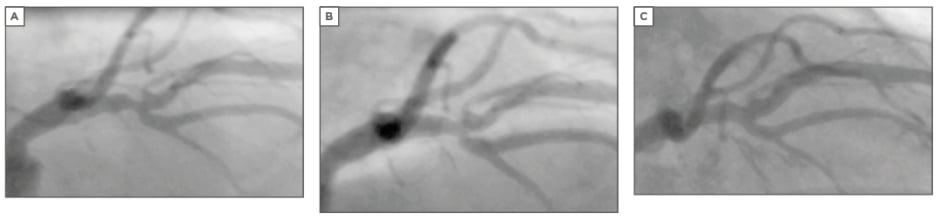
Figure 1: Proximal left anterior descending stenosis in bifurcation.
A) before, B) directly after, and C) 4 months after, showing late lumen enlargement. The bifurcation has been dilated and treated with the drug-coated balloon in both directions.
Peripheral Vessels
Positive remodelling after application of paclitaxel was found angiographically in peripheral vessels as well. More patients exhibited a late lumen gain in the DCB group when compared to the regular balloon group, and a small average late lumen gain (0.01 mm) was found in the PACIFIER trial,34 while there was an unexpected small average late lumen loss of 0.30 mm in the THUNDER trial.39 Although the clinical importance of positive remodelling in peripheral vessels may be less well-established and of minor importance in the large conduit vessels, the use of DCB in peripheral arterial disease is generally very well accepted as an anti-renarrowing strategy.
Drug-Coated Balloons in De Novo Trials
This review does not intend to summarise the clinical trials performed in de novo vessels with DCB. Despite a large number of patients in three small randomised trials, and in a number of large registries on mostly small vessels and bifurcations in >3,500 patients, there is a need for more data comparing DCB in de novo lesions to modern drug-ES. A large trial, however, is expected to be presented for the first time this year (BASKET small trial).40
LIMITATIONS
Dissections
One might consider the above observation as less relevant against the background of other reasons beyond restenosis necessitating stents, such as dissection and elastic recoil. While these considerations are worthwhile and valid, dissections are not a predictor of restenosis after PTCA and late lumen loss is less in B dissections than in A dissections.41 In addition, the overall number of PTCA cases that urgently require a stent is considered to be low. In the BENESTENT study,42 the rate of dissection ≥C according to National Heart, Lung, and Blood Institute (NHLBI) classification was <5%, and >70% of all cases could be randomised successfully to DCB versus stenting in the ongoing Basel small vessel study (Scheller, Nov 2017, personal communication).40 Therefore, a majority of percutaneous coronary intervention cases can benefit from the positive remodelling mechanism, while a subgroup, to be further defined, requires stents.
Thrombosis Rate
Another common claim is that stents are needed to prevent acute occlusion. However, de novo lesions treated only with DCB and achieving a result with residual stenosis ≤30% without major dissection and TIMI III flow exhibit no increased thrombosis rate. The criteria, achieved by an optimal PTCA result and considered to be safe, have been proposed by the Cadillac study43 and by the German Drug-Coated Balloon Consensus Group.44 Indeed, neither early nor late cases with acute or sub-acute thrombosis can be found among the >3,500 published cases in studies and registries using DCB as a stand-alone procedure. Therefore, this point of view also reveals a clinically significant option to achieve positive vessel remodelling in a large subgroup of patients, probably even in the majority of cases, by not using stents.
Bias by Patient Selection
The reported incidence of positive remodelling is limited to the patients that were suitable for DCB angioplasty. Thus, patients with major dissections (Class ≥C according to the NHLBI classification) were excluded, as were patients with major elastic recoil. The reported results can therefore only be applied to patients with a decent predilatation result. Since this is a large subgroup, and may represent the majority of patients, the observation is nevertheless meaningful and clinically relevant.
SUMMARY
There is strong evidence that positive vessel remodelling can serve as a good basis for a paradigm change in percutaneous coronary intervention towards fewer foreign body implants, obviating long-term problems with drug-ES, such as stent fracture, late malapposition, and late and very late thrombosis, as well as neoatherosclerosis in patients that have a decent acute PTCA result. A further and sustained benefit seems achievable in a large subgroup of patients. Some questions remain as to the potential benefit of this approach in various subgroups of patients and lesions, in severely diffuse disease and in milder disease stages, as well as in regard to the sustainability of long-term results after 5–10 years.