Abstract
Andexanet alfa is a modified factor Xa (FXa) drug designed to bind and sequester FXa inhibitors and thus reverse anticoagulation. The oral presentation reviewed in this article, presented by Dr Genmin Lu at the European Society of Cardiology (ESC) Congress 2018, provides new insights into the effect of the interaction between andexanet alfa and tissue factor (TF) pathway inhibitors on the restoration of thrombin generation (TG) in the TF (extrinsic) and non-TF (intrinsic) coagulation pathways.
INTRODUCTION
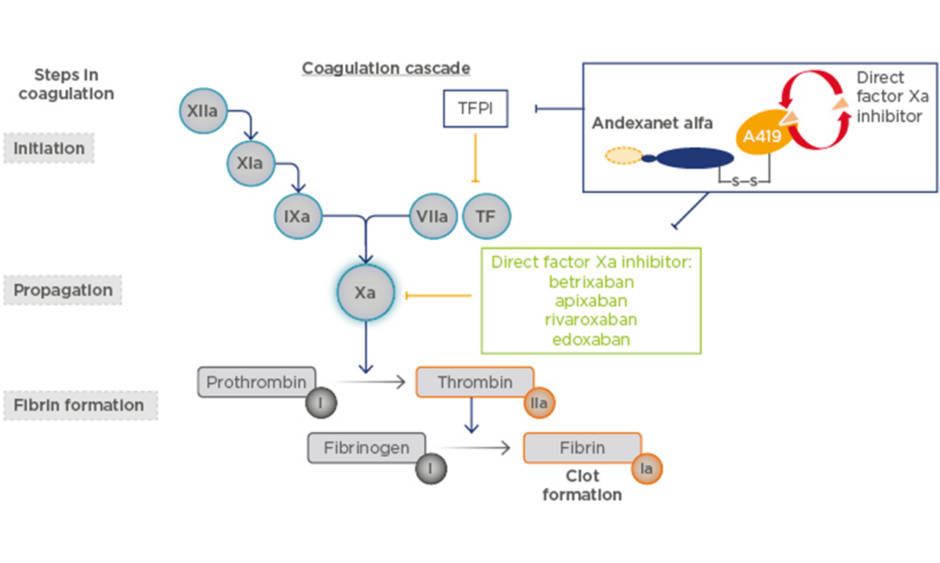
Andexanet alfa is a recombinant modified FXa protein with no enzymatic activity, designed to bind and sequester FXa inhibitors and thus reverse anticoagulation (Figure 1).1 Phase III studies have shown that andexanet alfa reverses the anticoagulation effects of the FXa inhibitors apixaban and rivaroxaban in older (aged 50–75 years), healthy volunteers.2 The ongoing ANNEXA-4 study in patients who had acute major bleeding after the administration of an FXa inhibitor showed 79–83% haemostatic efficacy.3,4 In 2018, andexanet alfa was approved by the U.S. Food and Drug Administration (FDA) for patients treated with rivaroxaban and apixaban when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.5 At the time of this oral presentation, andexanet alfa was under review for this indication in Europe.
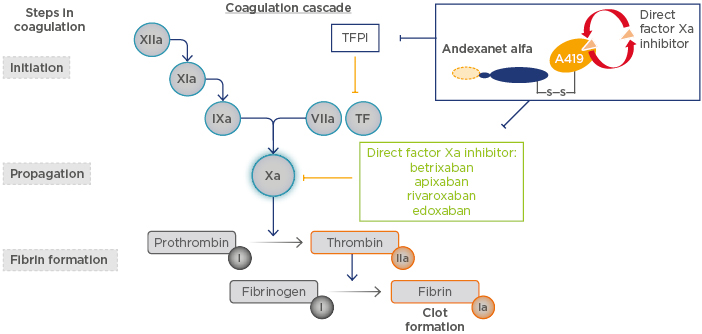
Figure 1: Andexanet alfa mechanism of action.
TF: tissue factor; TFPI: tissue factor pathway inhibitor.
ANDEXANET ALFA REVERSES THE ANTICOAGULATION EFFECTS OF APIXABAN AND RIVAROXABAN
The Phase III ANNEXA-A (apixaban) and ANNEXA-R (rivaroxaban) studies evaluated the effects of andexanet alfa versus placebo on healthy, older volunteers treated with 5 mg of apixaban twice daily or 20 mg of rivaroxaban daily, respectively. In Part 1 of the studies, subjects received andexanet alfa as a 400 mg (ANNEXA-A) or 800 mg (ANNEXA-R) bolus, whereas subjects in Part 2 received the same bolus plus a 480 mg (4 mg/min, ANNEXA-A) or 960 mg (8 mg/min, ANNEXA-R) infusion of andexanet alfa.2
When administered as a bolus or a bolus plus 2-hour infusion in subjects receiving anticoagulation for 4 days, andexanet alfa immediately and significantly reversed apixaban or rivaroxaban-associated anti-FXa activity. The reversal effect was sustained throughout the infusion and for approximately 2 hours following the end of infusion compared with placebo. Andexanet alfa treatment also reduced unbound, pharmacologically active concentrations of both FXa inhibitors and restored TF-initiated TG (TF-TG) compared with placebo. No serious adverse or thrombotic events were reported.2
AN ANDEXANET ALFA-TISSUE FACTOR PATHWAY INHIBITOR INTERACTION CONTRIBUTES TO THE SUSTAINED REVERSAL OF TISSUE FACTOR-INITIATED, BUT NOT NON-TISSUE FACTOR-INITIATED, THROMBIN GENERATION
As a modified FXa protein, andexanet alfa has no known major interactions with any other coagulation factors except for TF pathway inhibitor (TFPI), an endogenous, reversible inhibitor of the TF pathway. The interaction between andexanet alfa and TFPI may affect TF-TG (Figure 1), which is a secondary pharmacodynamic endpoint used in andexanet alfa clinical studies.2 The objective of the current study was to compare the effect of the andexanet alfa–TFPI interaction on restoration of TG in the TF (extrinsic) versus non-TF (intrinsic) pathways in the Phase III studies ANNEXA-A and ANNEXA-R.6,7
Retained plasma samples from subjects who received the andexanet alfa bolus plus 2-hour infusion were analysed using a validated non-TF-TG assay; this was similar to the TF-TG assay but instead used an activated partial thromboplastin time reagent (Actin FS; Siemens, Munich, Germany) as an activator. Restoration of TG was assessed by endogenous thrombin potential (ETP) and other TG parameters, including lag time, peak thrombin, time-to-peak, and velocity index.6,7 Following anticoagulation for 4 days, andexanet alfa significantly reduced apixaban and rivaroxaban-induced inhibition of both TF-TG and non-TF-TG versus placebo, as assessed by ETP and all other TG parameters (TF all p<0.0001; non-TF all p<0.001; Figure 2).6,7
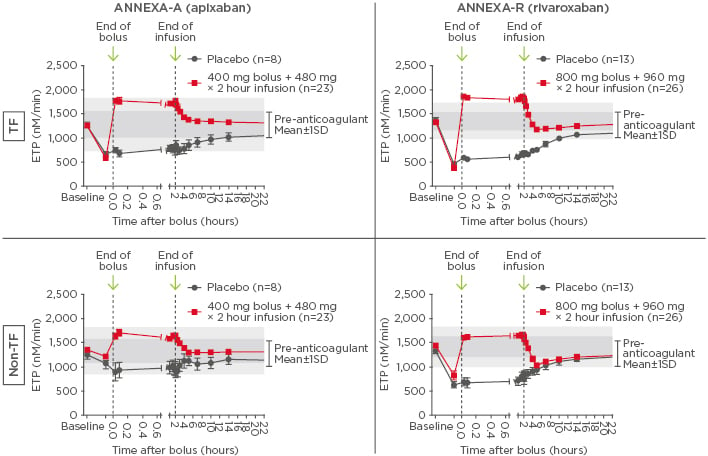
Figure 2: Time courses of tissue factor and non-tissue factor-initiated thrombin generation before and after administration of andexanet.
Andexanet versus placebo p values: TF: p<0.0001; non-TF: p<0.001. The shaded areas represent Day 1 pre-anticoagulant baseline ranges shown as mean±1SD (dark grey) and mean±2SD (light grey).
ETP: endogenous thrombin potential; SD: standard deviation; TF: tissue factor.
In apixaban-treated subjects, andexanet alfa restored TG to pre-anticoagulant levels under both TF and non-TF conditions, as assessed by ETP. It restored non-TF-TG in 100.0% of subjects (n=23) versus 37.5% of subjects who received placebo (n=8; p=0.0003).6 During administration of andexanet alfa, non-TF-TG had less of an increase in ETP above the pre-anticoagulant baseline level used to define the normal range compared with TF-TG. Following the end of the andexanet alfa infusion, there was a significant difference in TF-TG ETP between andexanet and placebo-treated subjects for at least 12 hours; however, for non-TF-TG, the ETP for andexanet-treated subjects decreased to that of the placebo- treated group within 2 hours after infusion and stayed within the pre-anticoagulant baseline range (mean±1 standard deviation).7
The results were similar in rivaroxaban-treated subjects. Andexanet alfa restored non-TF-TG in 100.0% of subjects treated with andexanet alfa (n=26) versus 15.4% of subjects who received placebo (n=13; p<0.0001).6 Non-TF-TG in the andexanet group had less of an increase in ETP above the pre-anticoagulant baseline level compared with TF-TG. Also, there was a significant difference in TF-TG ETP between andexanet and placebo-treated subjects for at least 12 hours after infusion; whereas for non-TF-TG, the ETP in the andexanet group decreased to that of placebo within 2 hours and gradually returned to the pre-anticoagulant baseline range (mean±1 standard deviation).6,7
CONCLUSION
Sequestration of the FXa inhibitors apixaban and rivaroxaban is the major contributor in restoring TG both during and immediately after the administration of andexanet alfa. The interaction between andexanet alfa and TFPI may contribute to the duration of the sustained reversal of TF-TG but does not contribute to non-TF-TG. Importantly, andexanet alfa restored TG to pre-anticoagulant levels under both conditions, whether TG was initiated via the extrinsic or intrinsic pathways. Overall, these data from studies in healthy volunteers suggest that the major function of andexanet alfa is to bind and reverse the anticoagulant activity of FXa inhibitors.
QUESTION AND ANSWER SESSION
A clinical concern for anticoagulant antidotes is ‘rebound’ when treatment is stopped. Have you experienced any rebound anticoagulation activity when treatment with andexanet alfa is stopped?
Andexanet alfa had an immediate and sustained effect on FXa inhibitors throughout and for approximately 2 hours following the end of infusion compared with placebo. The time course profile for the reversal of anti-FXa activity is consistent with the mechanism of action (binding and sequestering FXa inhibitors) and the effective half-life of andexanet alfa (~1 hour). However, as andexanet is cleared after the end of the infusion, the anticoagulant anti-FXa levels return to that of the placebo group. The andexanet alfa bolus followed by a 2-hour infusion was sufficient to completely reverse the anticoagulant effect, as previously demonstrated by TG and clinical efficacy in the ANNEXA-4 study in bleeding patients.
Following administration of the andexanet alfa bolus, what is the recommended period of treatment for the maintenance infusion? Is it until the bleeding clinically stops or do you use a prespecified timeframe?
We have evaluated an andexanet alfa bolus and bolus followed by infusion in Phase II and III studies in healthy volunteers. The two dose regimens shown here were sufficient for reversal of FXa inhibitors based on PD markers. Based on our data from animal models and ongoing Phase IV studies in bleeding patients, a 2-hour infusion is sufficient to stop bleeding in most patients.
The andexanet doses were different for the two inhibitors, apixaban and rivaroxaban. Was this due to the difference in plasma protein binding?
The different protein binding of apixaban and rivaroxaban is a minor factor when determining the doses of andexanet. The major difference is their volume of distribution. As andexanet binds to the inhibitor with high affinity in a 1:1 molar ratio, administration of andexanet causes redistribution of the inhibitor from the extravascular compartment into the plasma compartment. Rivaroxaban has an approximately 3-fold higher volume of distribution versus apixaban, hence the need for higher dosing to bind and sequester the total anticoagulant activity in the plasma.