INTRODUCTION
Acneiform rash has a great influence on patient quality of life. Such severe dermatologic reactions are induced by epidermal growth factor receptor (EGFR) inhibitors in antineoplastic therapy, which may lead to its future replacement as a treatment. Acneiform rash is based on specific underlying inflammation,1 and the severity of the skin reaction is based on the drug dosage and level of antitumour activity.2-4 Management of this side effect is therefore particularly significant.
METHODS
This study analysed 35 patients with Grade I-II acneiform rash, divided into 2 groups. All the participants received oral doxycycline (100 mg) twice a day for 10 days, and a local combination of fusidic acid and betamethasone valerate (20 mg/g and 1 mg/g cream) for 3 days in the morning, as well as various topical treatments in the evening for 3 months. Group 1 were treated with metronidazole 1% gel, while Group 2 were treated with ivermectin 1% cream. The Acne Dermatology Index (ADI) and Dermatology Life Quality Index (DLQI) were used to evaluate treatment results.
RESULTS
After the first week of oral doxycycline treatment with fusidic acid and betamethasone valerate, both groups showed evident regression to acneiform rash eruption. Group 1, who were treated with metronidazole 1% gel, subsequently showed a moderate response to treatment. Group 2, treated with ivermectin 1% cream, later showed a more rapid regression of both ADI and DLQI (Figure 1), with regression to acneiform rash eruption.
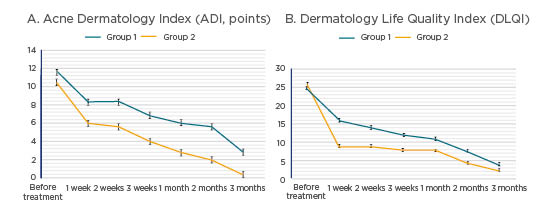
Figure 1: Dynamics in patients with acneiform rash before and after various methods of skin toxicity therapy.
CONCLUSIONS
Treatment with oral doxycycline at the early stages of Grade I-II acneiform rash led to significant clinical impact by preventing further dermatological aggravation and eruption. A combined treatment method of oral doxycycline with simultaneous application of 1% ivermectin cream, alongside fusidic acid and betamethasone valerate cream, appears to result in a faster clinical effect in comparison to combination treatment of oral doxycycline, fusidic acid and betamethasone cream, and 1% metronidazole gel. Combined treatment of Grade I-II acneiform rash with 1% ivermectin appears optimal for rapid improvement of clinical presentation and patient quality of life, whilst also allowing continuation of a patient’s main treatment scheme without interruption and dosage reduction. The increasing use of EGFR inhibitors in clinical practice highlights the importance of research into dermatologic toxicity, possible complications, and differential diagnosis, ensuring the right treatment is used. Prevention and early treatment in this group of patients is of great importance as it results in a greater chance of patient compliance in treatment of oncological diseases, and significantly improves patient quality of life.







