BACKGROUND
Immune checkpoint inhibitors and mTOR inhibitors are known to be associated with skin toxicity. mTOR inhibitors most commonly cause aphthous stomatitis, papule-pustulous dermatitis, and impaired wound healing.1 Programmed cell death protein receptor (PD-1) inhibitors confer increased risk of lichenoid reactions, pruritus, and maculopapular lesions.2 There has been an increased interest in studying idiosyncratic drug reactions arising during sequential treatment with immune checkpoint inhibitors and targeted immunotherapy drugs.3 Here, the authors describe the case of an acute maculopapular eruption in a patient treated with nivolumab and everolimus.
CASE DESCRIPTION
A 69-year-old female patient diagnosed with renal cancer was treated with nine cycles of the multitargeted receptor tyrosine kinase inhibitor sunitinib (50 mg per day orally). The patient was subsequently prescribed nivolumab (3 mg/kg per day intravenously). Remission had not been achieved, and she was sequentially treated with everolimus (10 mg per day intravenously). Under treatment with everolimus, patient examination demonstrated diffuse maculopapular eruption with excoriations (Figure 1). Her body surface area index was 67%. She also complained of severe generalised pruritus, with 10 points on the pruritus severity scale. Treatment with systemic prednisone and combination topical therapy was effective.
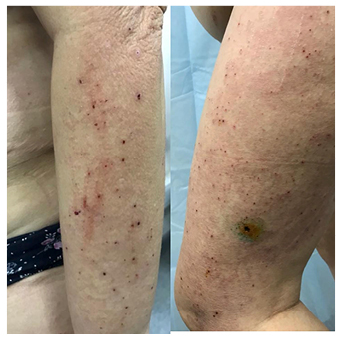
Figure 1: Acute maculopapular eruption with excoriations, induced by sequential treatment with nivolumab and everolimus.
DISCUSSION
This case represents the idiosyncratic drug reaction caused by sequential treatment with an immune checkpoint inhibitor and mTOR inhibitor. This type of drug toxicity stems from a double blockade of common immunogenic pathways. Paradoxical immune hyperactivity caused by this process is called ‘paradoxical activation’.4,5 This process underlying immunogenic skin toxicity requires further investigation, which may contribute to establishing patient management guidelines as well as developing proper preventive measures.
CONCLUSION
Combination cancer chemotherapy may induce idiosyncratic skin reactions. Patients sequentially treated with an immune checkpoint inhibitor and mTOR inhibitor may present with acute maculopapular eruptions and generalised pruritus. The described condition usually responds to systemic glucocorticoid therapy and topical combination therapy. Supportive treatment in patients with cancer includes early recognition and proper treatment of idiosyncratic drug reactions. Therefore, further investigations in this field would help provide optimal patient care without reducing and cancelling the anticancer therapy regimen.







