Support: The publication of this article was funded by Novartis Pharma AG.
Disclaimer: The views and opinions expressed are those of the authors and not necessarily those of Novartis Pharma AG.
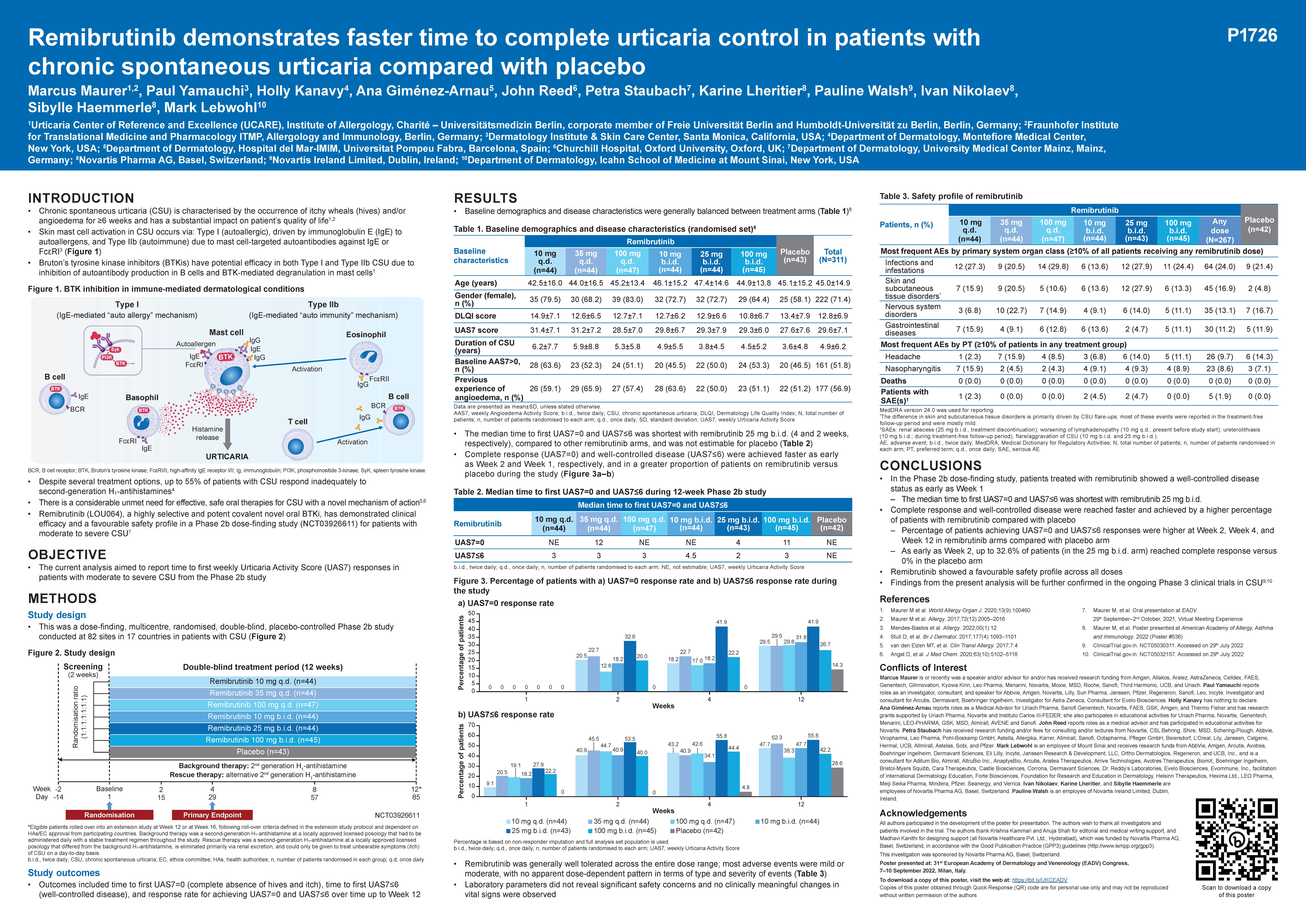
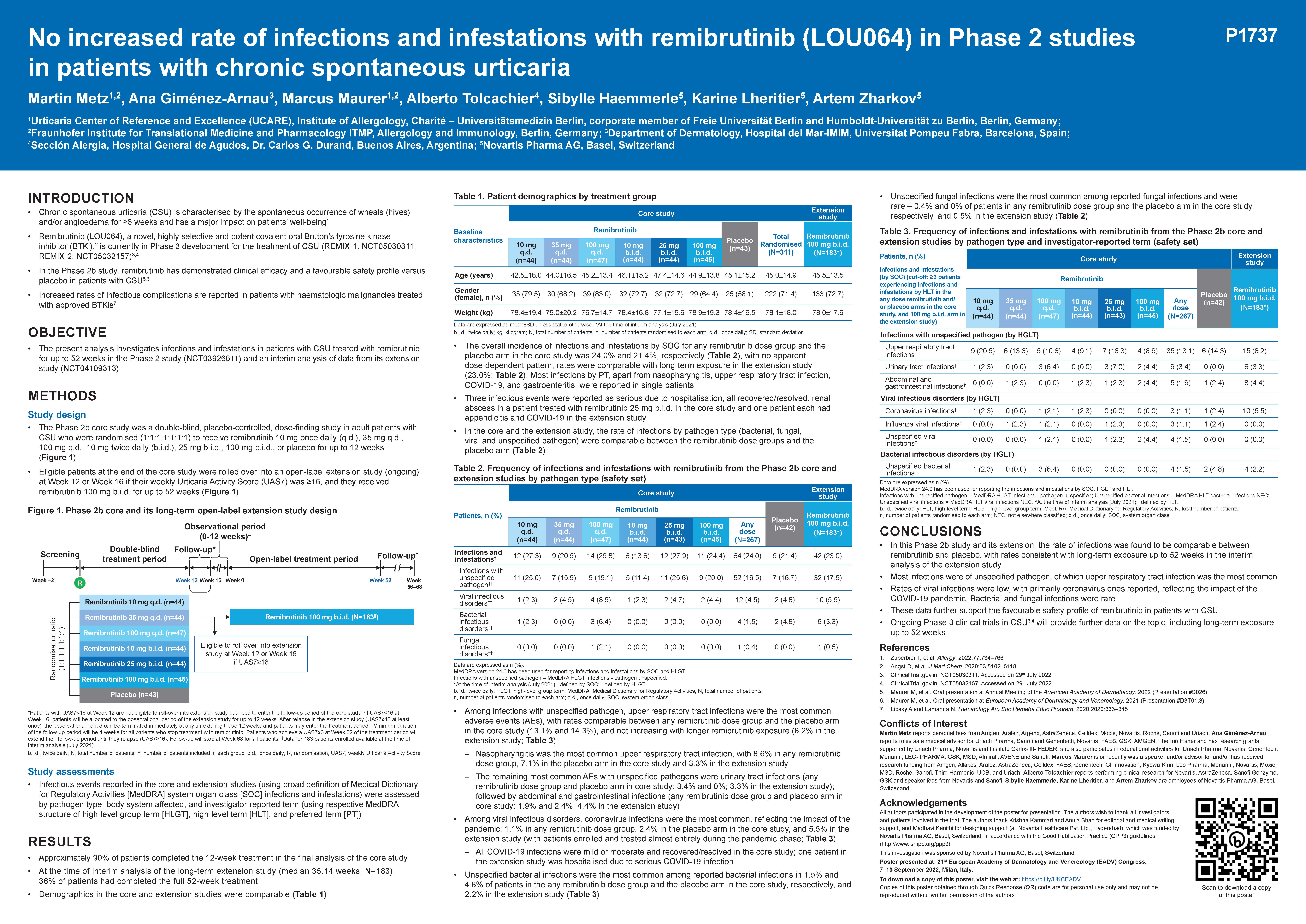
As part of the 31st European Academy of Dermatology and Venereology (EADV) Annual Congress held in Milan, Italy, and online, 7th–10th September 2022, five poster presentations outlined results from the Phase IIb study of remibrutinib (LOU064) in patients with chronic spontaneous urticaria inadequately controlled with H1-antihistamines. These posters presented data on quality of life, the need for antihistamine rescue medication to address symptoms, the time to complete urticaria control, and the safety profile of remibrutinib. The findings were outlined in these poster presentations and are summarised here.
Click here to view PDF
Click here to view PDF
Click here to view PDF
Click here to view PDF
Click here to view PDF