Abstract
Aim:
Chronic kidney disease is a non-communicable disease, and is the sixth fastest growing contributor of morbidity and mortality. Hemodialysis is one of the important therapeutic modalities that can improve survival in these patients, and can increase their life expectancy, but the cutaneous disorder can precede or follow the initiation of hemodialysis.
Methods:
This is a retrospective, observational study with a sample size of 250 patients, with a glomerular filtration rate <60 mL/min/1.73 m2 for a minimum duration of 3 months or more, undergoing hemodialysis. Patients post-renal transplant, HIV-positive cases, and pregnant patients were excluded from the study. Studied cases were recruited equally into two groups: the dialytic group (Group A) and non-dialytic group (Group B).
Results:
In the authors’ study, the prevalence of dermatological manifestations was 79% in the dialytic group and 75% in the non-dialytic group. The most common finding overall was xerosis (58%), which was more common in the dialytic (66%) group, both in number of patients and severity. The second most common finding was pallor (55%), which was seen more in the dialytic group (60%). Other major findings were pruritus (49%) and hyperpigmentation (37%). The intensity of pruritus was higher in non-dialytic patients. Specific cutaneous manifestations, such as Kyrle’s disease, were seen only in eight patients. Skin infections were seen in 17% of patients overall, and there was no major difference seen in both groups. The prevalence of nail findings, mucosal changes, and hair changes was also high in the dialytic group. Other specific cutaneous manifestations, like calciphylaxis, uremic frost, and nephrogenic fibrosing dermopathy, were not seen in the authors’ study. Hemodialysis has increased the life expectancy of patients with end-stage renal disease and has also brought about a rise in the number of manifestations, by giving time for these changes to occur. The severity of symptoms was also higher in patients on dialysis. This could be because of the higher mean duration of disease in the dialytic group compared to the non-dialytic group.
Conclusion:
Dermatological manifestations of chronic kidney disease were significantly associated with the mean duration of disease, which was higher in patients on dialysis. There was a higher prevalence of non-specific dermatological findings, such as xerosis, hyperpigmentation, nail findings, hair, and mucosal changes in the dialytic group, except pruritus. Any such cutaneous marker in the absence of a primary dermatological problem warrants a thorough search, including blood, urine, and radiological investigations, to rule out kidney disease.
Key Points
1. By analyzing dermatological signs and symptoms in patients with chronic kidney disease (CKD), the research aims to identify these manifestations as potential biomarkers for disease severity. This could lead to earlier detection and intervention, improving patient outcomes.2. By comparing dermatological symptoms before and after hemodialysis, the research aims to inform clinicians about the holistic management of patients with CKD, to improve their overall wellbeing.
3. Healthcare professionals can adopt a multidisciplinary approach by recognizing the relationship between skin manifestations, disease severity, and hemodialysis. Integrating dermatological assessments into routine evaluations can improve diagnostic accuracy and optimize treatment, leading to an enhanced quality of life for individuals with CKD.
INTRODUCTION
Chronic kidney disease (CKD) is a non-communicable disease that currently widely affects around 850 million people. Nowadays, it is an important public health issue, as it is the sixth fastest growing contributor of morbidity and mortality.1 Hemodialysis is one of the important therapeutic modalities which can improve survival in these patients, and can increase their life expectancy.2 Approximately 50–100% of patients with CKD have at least one associated non-specific cutaneous change.3,4 These cutaneous changes can precede or follow the initiation of hemodialysis therapy, and there are higher chances of developing newer cutaneous changes during the cycles of hemodialysis therapy. Pruritus is one of the most common characteristic symptoms of CKD, and is more severe in patients who are not on maintenance hemodialysis.5 Proposed etiologies for pruritus in CKD include changes related to xerosis, uremia, calcium and phosphate dysregulation, mast cell proliferation with high levels of histamine, hormonal derangement, and hypovitaminosis D.6,7 Other skin manifestations which are peculiar to dialysis are local complications, i.e., extravasation of blood, phlebitis, bacterial colonization of cannula, septicemia, allergic contact dermatitis to disinfectant, antiseptic solutions, and tape or bandages used to secure dialysis cannula or tubing.8,9 Complications associated with hemodialysis are bullous dermatosis of hemodialysis and gynecomastia.10
These cutaneous manifestations can precede or follow the initiation of hemodialysis therapy, and there are higher chances of developing newer cutaneous changes during the cycles of hemodialysis therapy, which may further affect the quality of life. The present study was conducted to evaluate the early cutaneous signs, and their prevalence and correlation, with the severity of disease in patients with CKD with or without hemodialysis, for early recognition of cutaneous signs, and to relieve further suffering, and decrease morbidity. The second objective of the study was to study the effect of hemodialysis on cutaneous manifestations.
PATIENTS AND METHODS
The retrospective, observational study was conducted in a multispecialty tertiary healthcare center from January 2017–June 2018 (total of 18 months). Taking the prevalence of cutaneous manifestations among patients with CKD as 80%,5 and absolute error of 5%, the sample size comes out to be 250.
Inclusion criteria for cases selected were as per the CKD definition, which is defined as kidney damage or glomerular filtration rate <60 mL/min/1.73 m2 for a minimum duration of 3 months or more, undergoing hemodialysis irrespective of the cause, of either sex, in the age group of 10–90 years, and attending the Nephrology and Skin department. Patients post-renal transplant, HIV-positive cases, and pregnant patients were excluded from the study. Cases were recruited in the study and divided into two groups, the dialytic group (Group A) and the non-dialytic group (Group B), to study and compare dermatological manifestations in patients with CKD. Group A included a total of 125 patients with CKD on maintenance hemodialysis (pre-dialysis criteria for hemodialysis: serum creatinine: >4 mg/dL; blood urea >70 mg/dL; serum potassium >6.5). Symptomatic patients, irrespective of the above-mentioned criteria, were considered for dialysis. The non-dialytic group included 125 patients with CKD. Detailed history and thorough physical examination, including a detailed assessment of skin, nails, hair, and mucous membrane for specific and non-specific cutaneous manifestations of CKD, were done in both groups and recorded on a predesigned proforma developed based on Skindex questionnaires. The Skindex questionnaire has 16 questions, but the authors opted for simpler modified question instruments based on Skindex-10, with their relevant subdomains for the patients on hemodialysis. Patients on maintenance hemodialysis were examined for any puncture marks, extravasation, and arteriovenous shunt dermatitis. Glomerular filtration rate was calculated for each patient using the Cockcroft–Gault formula, and staging of the severity of CKD was done accordingly. Various investigations were done, such as complete blood count, random blood sugar, renal function test, and serum calcium and phosphorous tests. Investigations such as Gram stain, Tzanck smear, potassium hydroxide mount, bacterial culture and sensitivity, fungal culture, chest X-ray, and skin biopsies were done wherever indicated. Institute ethical committee approval, and written and informed consent from all participants were obtained before enrolling in the study.
STATISTICAL ANALYSIS
The data obtained were statistically analyzed using Microsoft Excel 2016 (Microsoft, Redmond, Washington, USA), and the statistical ꭓ2/Fisher exact test was used. The categorical variables were presented in number and percentage (%), and continuous variables were presented as mean±standard deviation and median. Quantitative variables were compared using the ꭓ2/Fisher exact test.
RESULTS
A total of 250 patients, primarily consisting of patients with CKD, and patients with CKD undergoing hemodialysis, were analyzed.
The groups were comparable, as there was no statistically significant difference between the sex, age, duration, CKD stage, and CKD etiologies. The mean hemoglobin level in both groups was 7.95±1.46 mg/dL in the dialytic group and 8.09±1.16 mg/dL in the non-dialytic group, and a maximum number of patients in the authors’ study had low hemoglobin levels. The mean blood urea level was 119.81±44.13 mg/dL in patients on dialysis and 115.50±42.45 mg/dL in non-dialytic patients. The most common underlying etiological cause of CKD in the studied participants was hypertension (36% in the dialytic group and 40% of the non-dialytic group), which was followed by diabetes (27% in the dialytic group and 22% in the non-dialytic group).
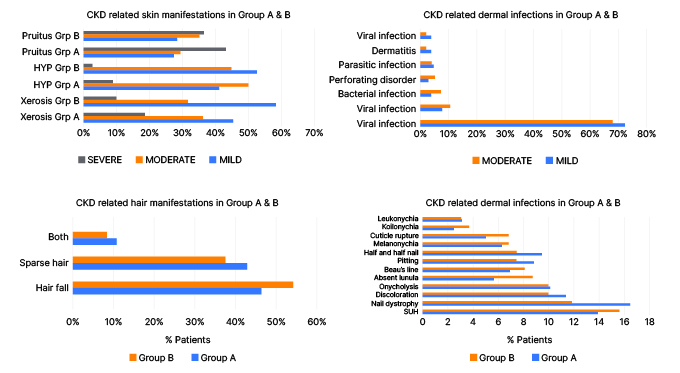
Figure 1: Chronic kidney disease-related skin, hair, and nail manifestations in patients with or without hemodialysis.
CKD: chronic kidney disease; HYP: hyperpigmentation; SUH: subungual hyperkeratosis.
Both hypertension and diabetes were found in 17.6% in the dialytic group and 16.0% in the non-dialytic group. In this study, most of the patients were in Stage 5 of CKD at the time of examination in both the dialytic (n=116; 92%) and pre-dialytic group (n=114; 91%). The mean duration of disease in dialytic and non-dialytic patients was 25.2 months and 12.4 months, respectively, with a wide variation in the duration of disease ranging from 5 months to 15 years. In the present study, nail dystrophy was the most common nail finding in patients on dialysis (n=26; 20%) followed by SUB (n=22; 17%), nail discoloration (n=18; 14%), and half and half nail (n=15; 12%); while in non-dialytic patients, subungual hyperkeratosis (n=25; 20%) was the most common nail finding, followed by nail dystrophy (n=19; 15%), nail discoloration, and onycholysis (n=16; 12%); Figure 1 and 2). In this study, the most common mucocutaneous finding was xerostomia (n=8; 6.40%) followed by macroglossia (n=6; 4.80%) in the analytic group, while in the non-dialytic group, the most common finding was macroglossia (n=6; 4.80%), followed by the fissured tongue (n=5; 4%; Figure 3). Xerosis was mild in most of the cases, and found more commonly in the dialytic group (n=86; 66.6%) in comparison to the non-dialytic group (n=60; 48.0%). Mild-to-moderate hyperpigmentation was seen in 56 (44%) dialytic cases and 38 (28%) non-dialytic cases. Moderate-to-severe pruritus was seen mainly in all non-dialytic patients (n=74; 58%), while only 40% of patients of the dialytic group were having pruritus. Pallor was seen commonly in both groups. Among specific skin findings in CKD, associated perforating disorders were seen in five patients (4.0%) in the non-dialytic group and three patients (2.4%) in the dialytic group (Figure 4). In this study, dermatological manifestations of CKD were significantly associated with the mean duration of disease, which was higher in patients on dialysis. There was a higher prevalence of non-specific dermatological findings such as xerosis, hyperpigmentation, nail findings, hair, and mucosal changes in the dialytic group, except pruritus. The other complication was dilation of the venous segment near the arteriovenous fistula, and eczema around the arteriovenous fistula, which was seen in 5.6% of cases.
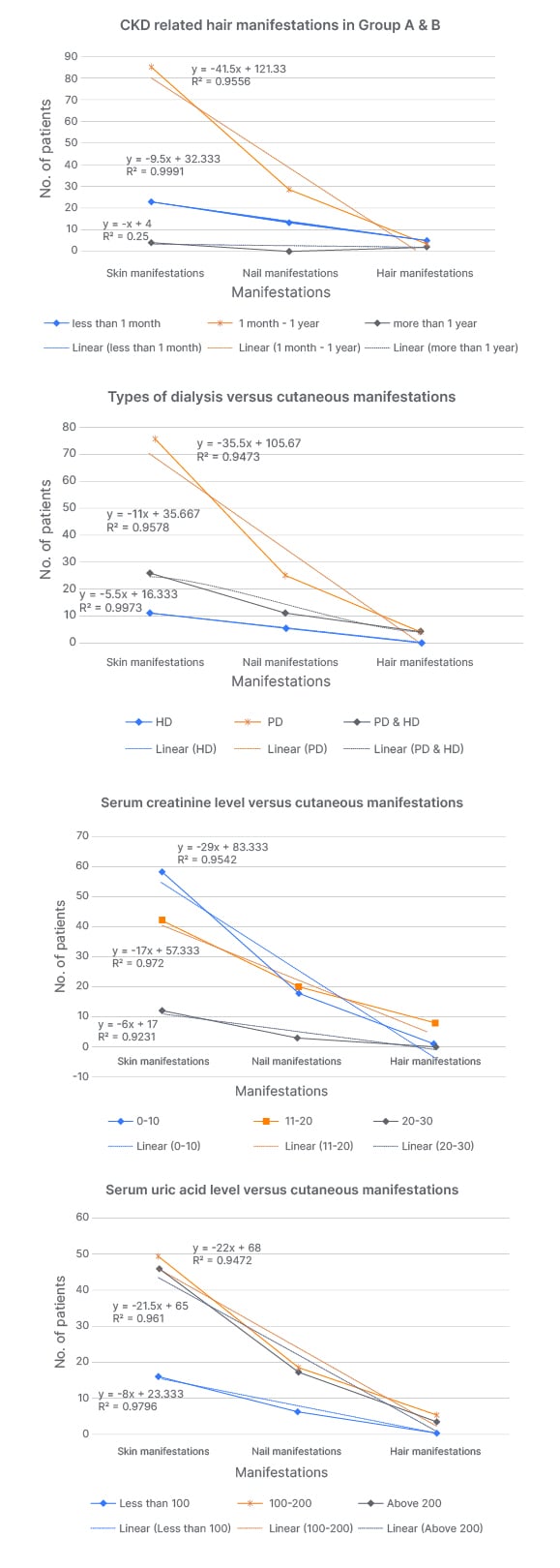
Figure 2: Correlation between duration and type of dialysis, serum creatinine, and uric acid level with cutaneous manifestations.
Values r2 >0.3 are considered statistically significant.
HD: haemodialysis; PD: peritoneal dialysis.
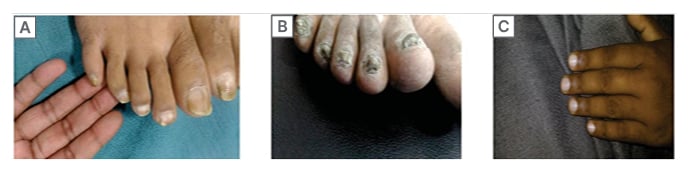
Figure 3: A) Lindsay’s half and half nails, a characteristic colored band over distal nail plate and pale proximal nail plate; B) nail dystrophy; C) absent lunula.
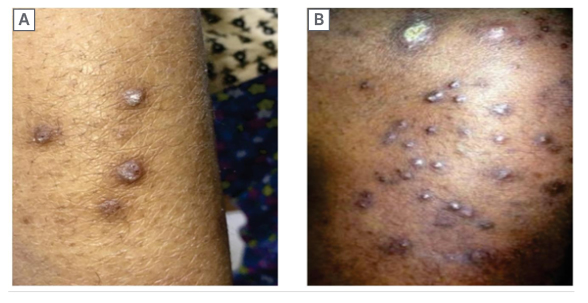
Figure 4: Perforating folliculitis with keratotic papules over leg (A) and abdomen (B). Note severe ichthyotic skin over the trunk.
CORRELATION
The authors observed that skin, nail, and hair manifestations showed a statistically significant correlation with the duration of dialysis (r2=0.9556 and 0.9910) during the first year of initiation of dialysis, while after 1 year this correlation becomes weaker (r2=0.2500). Cutaneous manifestation has an equally statistically significant correlation with all hemodialysis and peritoneal dialysis (r2=0.9473, 0.9578, and 0.9973). Similarly, there was a statistically significant correlation between the cutaneous manifestation with serum creatinine level (r2=0.9542, 0.9720, and 0.9230) and serum uric acid level (r2=0.9472, 0.9610, and 0.9910). This correlation is more significant in patients with serum creatinine level between 11–20 mmol/L and serum uric acid level above 200 mmol/L.
DISCUSSION
This retrospective observational study was done to study and compare dermatological manifestations in patients with CKD. In the present study, 79% of patients in the dialytic group, and 75% of patients in the non-dialytic group, had at least one dermatological manifestation. Similarly, in a study by Picó et al.,11 cutaneous manifestation was seen in all patients undergoing hemodialysis. Nunley et al.4 also found that 50–100% of patients with end-stage liver disease had at least one cutaneous lesion. In other Indian studies, the frequency of skin involvement ranged from 96–100%.5,12
In the authors’ study, xerosis was the most common non-specific manifestation noted, with an overall prevalence of 58%, with the dialytic group (66%) having a statistically significantly higher prevalence (P=0.211) in comparison to non-dialytic patients (48%). Xerosis mainly affects the upper and lower limbs, back, and face. Diffuse xerosis was observed in 11% of overall cases. Similarly, in a study done by Gilchrest et al.13 and Yosipovitch et al.,14 high prevalence of xerosis was observed in 69% and 62% of non-diabetic patients, respectively, and 70% and 91% of patients on hemodialysis, respectively.13,14 The higher prevalence of xerosis in the authors’ study could be due to non-availability and inappropriate use of moisturizers, due to poor economic status. Other factors associated with xerosis in the Indian scenario could be poor diet, protein malnutrition, tropical climate with more sun exposure, and chronic dehydration. The other possible etiological factors for xerosis include a reduction in the size and functional abnormality of eccrine sweat glands, causing epithelial dehydration and dehydration due to the use of chronic and high doses diuretics in patients with CKD.10,15 In the authors’ study, pallor was the second most common manifestation and observed in 55.20% of patients (dialytic group: 59.20%; non-dialytic group: 51.20%). No significant difference was found between the dialytic and non-dialytic groups. Similar findings were reported by Udayakumar et al.,5 Levillard et al.,16 Chanda et al.,17 and Pradhan et al.,18 who reported pallor in 60%, 70%, 57%, and 54% of patients with uremia, respectively.5,16-18 Chronic malnutrition superimposed on the anemia of chronic disease may have resulted in a higher prevalence of skin pallor. Pruritus was found in 49% of patients, with a higher prevalence in the non-dialytic group (59.2%) in comparison to the dialytic group (40.2%). The severity of pruritus varied in patients. Some patients gave a history of improvement of pruritus after dialysis. Similarly, a study by Chanda et al.17 reported a prevalence of pruritus higher in the non-dialytic group (60%) than the dialytic group (40%), similar to the authors’ study.17 The other studies reported prevalence of pruritus in the pre-dialytic period in patients ranging from 22% to as high as 85%.19,20 Proposed etiologies for pruritus in CKD include changes related to xerosis, uremia, calcium and phosphate dysregulation, mast cell proliferation with high levels of histamine, hormonal derangement, and hypovitaminosis D.6-7
In the authors’ study, hyperpigmentation was seen in 37% of patients, with a higher prevalence in the dialytic group (44.8%) than in the non-dialytic group (30.4%). Hyperpigmentation was more common in sun-exposed areas like the face and neck, while 30% of patients had diffuse hyperpigmentation. Skin manifestations were comparatively lesser in proportion, when compared to Picó et al.,11 Hajheydari et al.,3 and Udayakumar et al.,5 who reported pigmentary changes in 70.00%, 66.73%, and 53.00%.3,5,11 An increase in the amount of melanin in the basal layer of the epidermis is due to an increase in the concentration of the poorly dialyzable β-melanocyte-stimulating hormone.21,22
In the present study, skin infections were lesser in proportion than Udayakumar et al.5 and Sultan et al.,23 who reported a prevalence of 67% and 40% in their studies. The authors observed skin infections in 17.0% of the patients, 7.2% of which were fungal (tinea infection in 78% and pityriasis versicolor in 22%), 4.4% were bacterial infections (in form of pyoderma), 3.0% viral infections (herpes zoster), and 3.6% parasitic (scabies). There was no statistically significant difference between the dialytic and non-dialytic groups. The high incidence of infections might be due to lymphopenia, decreased B cell activity, alteration of the T cell subsets, and other disorders associated with diabetes, low albumin, elevated intracellular calcium, acidosis, or repetitive vascular procedures.24 CKD-associated specific skin manifestations include acquired perforating dermatosis, metastatic calcification, uremic frost, nephrogenic fibrosing dermopathy, and bullous dermatosis of dialysis. Among the acquired perforating dermatosis, in the authors’ study, Kyrle’s disease was noted in eight patients (three dialytic and five non-dialytic). Other specific manifestations, like perforating folliculitis and perforating collagenases, were not found in the authors’ study.
Nail changes observed in the authors’ study were more significant in the dialytic group (54%) than in the non-dialytic group (38%). Nail dystrophy (21%), followed by subungual hyperkeratosis (17%), and half and half nail (13%), were most commonly found in the dialytic group, while in the non-dialytic group, subungual hyperkeratosis was most common (20%), followed by nail dystrophy (15%) and half and half nail (9%). Nail discoloration (13%), pitting (10%), Beau’s lines (9%), onycholysis (12%), absent lunula (9%), melanonychia (8%), koilonychia (4%), and leukonychia (4%) were the other nail findings seen in cases. Similarly, Shrestha et al.,25 Chanda et al.,17 Pradhan et al.,18 Asokan et al.,26 and Udayakumar et al.,5 reported 21–62% nail changes in their studies. Half and half nail was the most peculiar finding in patients with CKD, and was reported in between 9–40%.27
Hair changes were observed in 20.8% of patients (dialytic: 22%; non-dialytic: 20%), with no statistically significant difference between the two groups. Hair fall (11%), and sparse hair distribution (8%) with dryness and discoloration of hair, were the most common cases overall. This was significantly low in comparison to a study by Hajheydari et al.,3 which reported >50% of the patients with at least one hair disorder, out of which the most common is disseminated hair loss of the entire body, in particular of the lower limbs (9%).3 Udayakumar et al.5 reported sparse hairs in 11% of cases.5 The cause of sparse hair may be attributed to decreased secretion of sebum. The mucosal changes were observed in 14% of total patients in the present study, with no statistically significant difference between the dialytic and non-dialytic groups.
A similar mucosal change pattern was reported by Udayakumar et al.5 and Pradhan et al.18 (13–15%). Pradhan et al.18 reported a significantly higher number of cases (39%) with mucosal changes. Xerostomia, macroglossia, fissured tongue, and angular cheilitis were the other changes reported.
In the authors’ study, local complications of dialysis were seen in 20.8% of the patients, while Masmoudi et al.28 and Chanda et al.17 reported local complications of dialysis in 33% and 74% of cases. In the authors’ study, the most common complication was dilation of the venous segment near the arteriovenous fistula, which was seen in 15% of cases, while Masmoudi et al.28 reported it in 22%.
Another complication was eczema around the arteriovenous fistula, which was seen in 5.6% of cases, and reported in 8.0% of patients with CKD by Udayakumar et al.5 Patients with diabetes as an etiological factor for CKD had a more significant prevalence of xerosis, pruritus, and hyperpigmentation. Attia et al.29 and Gilchrest et al.13 also reported the increased severity of skin manifestations in patients with CKD.13,29
However, their sample size was small (n=25), which was not statistically significant. In the authors’ study, cutaneous findings were higher in patients on dialysis than in the non-dialytic group, except for pruritus. With hemodialysis, life expectancy of patients with end-stage renal disease has increased, and has also brought about a rise in the number of manifestations, giving time for these changes to occur. The severity of symptoms was also higher in patients on dialysis. This could be because ofthe higher mean duration of disease in patients on dialysis compared to the non-dialytic group.
CONCLUSION
Based on the results of the present study, the authors propose that a routine skin examination of the patient with CKD receiving or not receiving hemodialysis is an efficient way of early detection of the CKD-related skin manifestations, and can improve care for the patients. A limitation of the present study is a small sample size, which limits generalizability. Another limitation of this study is related to the Skindex-10 questionnaire. In some cases, a non-applicable answer was given, which can lead to a false-negative result, potentially underestimating the impact of the disease. This was observed in 21% of patients on dialysis and 11% of patients with diabetes.






