Abstract
Acne vulgaris (AV) is a chronic inflammatory disease of the pilosebaceous unit. AV has a multifactorial pathogenesis with specific roles played by the sebaceous glands, abnormal follicular hyperkeratinisation, inflammation, Propionibacterium acnes, hormonal factors, immune mediators, and genetic and environmental factors. Significant improvements have been made to elucidate acne pathogenesis, through developments in molecular biology, immunology, and genetic techniques. Toll-like receptors and antimicrobial peptides play significant roles in the host defense system against different pathogenic micro-organisms on the skin and these molecules induce several immunological responses. It is well known that toll-like receptors and antimicrobial peptides play important roles in AV pathogenesis and further understanding of these will contribute to improvements in treatment.
INTRODUCTION
Acne vulgaris (AV) is a multifactorial disorder of the pilosebaceous unit and can affect individuals of all ages, but is particularly common among adolescents. AV is not a life-threatening disease, but it can negatively affect the mood state during adolescence and hence may reduce quality of life. AV is a chronic inflammatory disease, with several factors playing roles in its development.1 Increased sebum production, alteration of the quality of sebum lipids, regulation of cutaneous steroidogenesis, androgen activity, interaction with neuropeptides, exhibition of pro and anti-inflammatory properties, follicular hyperkeratinisation, and the proliferation of Propionibacterium acnes within the follicle may be listed among these factors.2 Although acne is the most common skin disease, its pathophysiology is complex and incompletely understood.3
The increased sebum production is a major simultaneous event associated with the development of acne. Lipids produced by sebaceous glands have a variety of effects on signal transduction and are involved in biological pathways of acne. Moreover, fatty acids act as ligands of nuclear receptors. Sebaceous gland lipids have pro and anti-inflammatory properties. Furthermore, some hormones, such as androgens, control the sebaceous gland size and sebum secretion.2
Molecular components called pathogen-associated molecular patterns (PAMPs) that exist as part of micro-organisms are known to have a significant role in the protection of immunity. The cells of the immune system recognise the PAMP receptors through pattern recognition receptors (PRRs); toll-like receptors (TLRs) are a member of the PRR class and each TLR recognises a different PAMP region.4 Activation of TLRs stimulates the production of antimicrobial effector molecules while inducing the signalling pathways that provide an increased adaptive response by facilitating the display of the costimulatory molecules and the release of cytokines at the same time. Moreover, TLRs activate the host’s defence mechanisms that defend against foreign organisms.5 Multiple cell types within the skin express TLRs. Keratinocytes and sebocytes of the epidermis were shown to contain functional TLRs.4 AV lesions have been associated with increased TLR2 and TLR4 expression. Moreover, P. acnes induces the release of pro-inflammatory cytokines from the monocytes through the activation of TLR2.5
By activating antimicrobial pathways, TLR activation results in the release of antimicrobial peptides (AMPs).6 AMPs consist of 10–50 amino acid residues and are positively-charged amphipathic molecules that are considered the natural antibiotics of the immune system. AMPs have several subtypes and various human epithelial tissues, including the epidermis, release them. AMPs produced in the skin to ensure elimination of micro-organisms protect the epithelium from microbial infections and colonisation. P. acnes colonisation, which increases in AV, is known to cause accumulation of inflammatory cells in the infected region, by increasing the release of chemokines from keratinocytes and mononuclear cells, as well as the secretion of human β-defensin (hBD)1 and hBD2.7 Cathelicidins are known to affect the functions of TLRs. P. acnes causes induction of cathelicidin expression in the sebocytes.8 The acne pathophysiology and the roles of TLRs and AMPs in acne pathogenesis are summarised in Figure 1. The present review focusses on the role and importance of TLRs and AMPs in AV aetiopathogenesis.
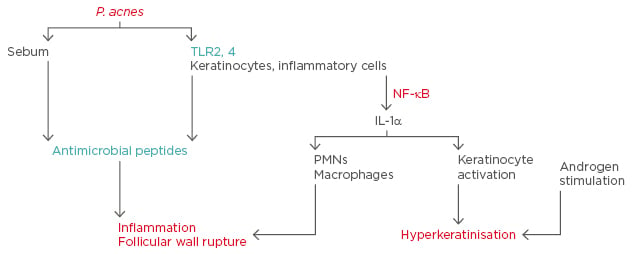
Figure 1: Acne pathophysiology and the roles of toll-like receptors and antimicrobial peptides in acne.
IL: interleukin; NF: nuclear factor; P. acnes: Propionibacterium acnes; PMNs: polymorphonuclear leukocytes; TLR: toll-like receptor.
ACNE VULGARIS AND TOLL-LIKE RECEPTORS
The human epidermis represents the first defence barrier of the host. The host response against pathogens is characterised by two types of immunity: innate and adaptive. In addition to playing a role in recognition and elimination of the pathogens, innate immunity also contributes to the development of adaptive immunity. It achieves these functions mainly through PRRs.9 The mechanisms involved in the recognition of the pathogenic agents by the host’s immune cells and generation of agent-specific immune responses have been investigated in more detail in recent years.10 The receptors that exist on immune cells and are responsible for recognising the pathogens were first discovered in 1996 in Drosophila melanogaster flies and they were then called ‘tolls’. Currently these molecules, which are interleukin (IL)-1-receptor-I homologues, are called TLRs.11 TLRs are transmembrane proteins of the PRR protein family and act through their immunoadjuvant abilities and by activating cells that display antigens.9 TLRs ensure the generation of a pathogen-specific immune response and this capability is genetically determined.12 The discovery of TLRs significantly contributed to the understanding of the mechanisms underlying the generation of cellular and hormonal immune responses in host/pathogen interactions.13
A TLR increases the levels of costimulatory proteins ensuring a more effective stimulation of T cells by dendritic cells.14 An innate immune response is primarily characterised by the functions of dendritic cells, monocytes, natural killer cells, lymphocytes, and epithelium cells; these cell types were all shown to express TLRs.12 So far, 11 types of TLRs have been defined and other than TLR10, the ligands of all TLRs are known.11 Multiple ligands have been identified for TLR2 and TLR4.15 Among the TLRs expressed in human keratinocytes, TLR2 and TLR4 are particularly important. TLR4 acts as a signal receptor for the lipopolysaccharides in Gram-negative bacteria. TLR2 recognises molecules with different structures, such as lipoproteins, lipopeptides, peptidoglycan, lipoteichoic acid, lipoarabinomannan, glycolipids, and zymosan.6 Additionally, TLR expression by cells is a dynamic process and certain ligands were shown to result in the activation of different TLR subtypes in different tissues.16
The studies investigating the roles of TLRs in AV are rather new. Kim et al.17 suggested that the local inflammatory response in acne results from the effects of cytokines released from the TLR2+ monocytes stimulated by P. acnes. In that study, TLR2+ macrophages were identified in the acne lesions and around pilosebaceous units, and their numbers were shown to increase during the course of the disease.17 Jugeau et al.6 reported elevated TLR2 and TLR4 expression in the epidermal keratinocytes in superficial inflammatory acne. In their study, TLR2 levels in the epidermal keratinocytes were higher than TLR4 levels. Similarly, TLR2 distribution was found to be associated with keratinocyte maturation.6
Keratinocytes activated by different P. acnes extracts show increased TLR2 and TLR4 expression in vivo. Expression of acne matrix metalloproteinase-16 (MMP16), IL-8, and hBD2 was also shown to be enhanced in the keratinocytes stimulated through TLR2 and TLR4.18 TLR2 and TLR4 are associated with the inflammatory reaction and tissue destruction in acne. These findings suggest that in the presence of inflammatory acne, P. acnes stimulates the keratinocytes and/or macrophages with the mediation of TLR.15
Chemokine and cytokine synthesis in human monocytes is induced by P. acnes-mediated TLR2 activation. TLR2-mediated pro-inflammatory cytokine release was suggested to worsen the disease course by causing inflammation and tissue destruction. There are also some studies arguing that TLR2 alone is sufficient for the activation of monocytes by P. acnes.19
In their study aiming to identify the role of P. acnes in inflammatory acne, Jugeau et al.6 demonstrated increased TLR2 and TLR4 expression in the epidermis of acne lesions in vivo. In addition, TLR2 and TLR4 expression in vitro was shown to be enhanced after the incubation of keratinocyte cultures by bacterial fractions and a higher amount of MMP9 was released from the keratinocytes in vitro.6 In another study, Selway et al.20 investigated the role of TLR2 in acne pathogenesis and its contribution to comedogenesis by using immunohistochemistry and Western blotting methods in ex vivo sebaceous gland and primary keratinocyte cultures. They demonstrated TLR2 expression in basal keratinocytes, infundibular keratinocytes, and sebaceous glands, and found that TLR2 activation in human keratinocytes increased IL-1α release in vitro. The increase in TLR2 activation was also shown to stimulate hypercornification and comedogenesis in human sebaceous glands ex vivo.20
Previous studies have demonstrated that certain medications used in acne treatment can alter TLR expression.21-26 Liu et al.21 detected that all-trans retinoic acid treatment can reduce the expression of TLR2 and its co-receptor CD14 but does not lead to a significant change in the expression of TLR1 or TLR4. In a study performed by Tenaud et al.22 in patients with acne, TLR2 expression in the keratinocytes was shown to be reduced after 24 hours of incubation with adapalene. Similarly, Jarrousse et al.23 demonstrated that zinc salts exert anti-inflammatory effects on acne by reducing the levels of TLR2.23 Dispenza et al.24 showed that the monocytes of patients with acne expressed more TLR2 and less TLR4 compared to healthy controls. Isotretinoin treatment, on the other hand, reduced TLR2 expression in the monocytes in vivo but did not alter TLR4 expression.24 Lee et al.25 evaluated the effect of magnesium ascorbyl phosphate on MMP9, TLR4, and AMP expression in sebocyte cultures by using reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) methods, and determined that magnesium ascorbyl phosphate treatment reduced MMP9, TLR4, and AMP expression. Jeong et al.26 investigated the effects of topical aminolevulinic acid photodynamic therapy on the levels of TLR2 and TLR4 expression. In that study 5 out of 10 cases had enhanced TLR2 expression before photodynamic therapy, and it was reported that although TLR2 expression decreased after therapy in patients with enhanced TLR2 expression, no change was noted in patients with normal TLR2 immunoreactivity. Similarly, TLR4 expression was found to be elevated before therapy in 3 out of 10 patients and the levels of TLR4 expression decreased after therapy in these patients.26
Clinically, AV can present with different non-inflammatory and inflammatory lesions. In the biopsy samples obtained from AV lesions TLR2 expression was found to be extensive, particularly in the perifollicular region, and long-term disease was associated with an increased number of TLR2+ cells.27 Moreover, a positive correlation was found between the severity of AV lesions and concentration of TLR2-expressing cells.28
A limited number of studies have been performed to date to investigate how TLR expression varies between different types of AV lesions and the different regions of a lesion. Bakry et al.29 examined 30 acne cases (involved and non-involved skin) and the normal skin biopsies of 30 healthy controls using immunohistochemical methods. In this study, there were significant differences between acne-involved skin and normal skin, and between acne-involved and non-involved skin, concerning TLR2 expression intensity in pilosebaceous units and dermal inflammatory infiltrates. TLR2 expression was shown to be more intense in the pilosebaceous units and dermal infiltrates regions of inflammatory and severe acne lesions.29 In a previous study, we evaluated the levels of TLR2 and TLR4 expression in comedonal, papular, pustular, and nodular lesions of AV according to different regions of the lesion including the epidermis, site of inflammation, dermis, and skin appendages. We found that TLR2 expression was elevated in the papular and comedonal lesions compared to the nodular lesions in the epidermis region, and in the papular lesions compared to pustular lesions in the inflammation and dermis sites. Moreover, TLR4 expression was lower in the comedonal lesions than papular lesions in the dermis.1 These findings suggest that the TLRs play important roles in the development of different clinical lesions of AV.
ACNE VULGARIS AND ANTIMICROBIAL PEPTIDES
The epidermis not only acts as a physical barrier against infectious agents, but it also quickly develops resistance mechanisms. This rapid response is regulated by certain low-molecular weight proteins called AMPs. AMPs are an important component of our innate immune system. AMPs are structures that both provide the baseline protection and also induce the development of the adaptive immune system. AMPs are recognised based on their cutaneous antimicrobial and immunomodulatory characteristics and inhibit bacterial, fungal, protozoal, and viral infections.30 These proteins are considered to perform their antimicrobial functions by binding to the surface of microbes and forming pores on their cell membranes.31 The AMP family consists of α and β-defensins, cathelicidins, S100 proteins, ribonucleases, and others.30 These small endogenous cationic proteins are found in the keratinocytes, eccrine glands, mast cells, phagocytes, and sebocytes. In resting skin cells, so far it has been identified that β-defensins (hBD1, hBD2, hBD3), cathelicidin (LL37), psoriasin, and ribonucleases are all synthesised by the keratinocytes, while dermcidin is a molecule of the human sweat. AMP release may be induced by bacteria, bacterial products, TLRs, or pro-inflammatory cytokines.31 In addition to their antimicrobial effects, AMPs are also significantly involved in inflammatory cell migration and cytokine release.32 Due to their cationic characteristics they frequently act by inducing a negative charge on bacterial membranes.30
Several studies performed in recent years have indicated that AMPs play significant roles in the pathogenesis of chronic inflammatory skin disorders.33 The regulation of the synthesis of innate and specific AMPs is known to be impaired in AV patients.34 The roles of AMPs, particularly those including hBD and cathelicidin subgroups, have been extensively investigated in AV pathogenesis.34,35 Defensins are a small AMP family, and are expressed in all human epithelial tissues.7 Three subtypes of β-defensins have been identified thus far, hBD1, hBD2, and hBD3, and they were shown to be expressed by several epithelial cells.36 β-defensin release increases in response to microbial or pro-inflammatory stimuli.37 Typical fatty acids found in sebum, such as palmitic acid and oleic acid, were shown to increase the antimicrobial activity of the sebocytes against P. acnes by enhancing hBD2 expression.38
Chronnell et al.34 evaluated hBD1 and hBD2 expression patterns in the pilosebaceous units of healthy individuals and AV patients and reported a marked increase in hBD1 and hBD2 expression in AV patients, particularly in the all suprabasal layers of the epidermis, the distal outer root sheath of the hair follicle, and the pilosebaceous duct. That study indicated a marked elevation in hBD1 and hBD2 expression in the skin with lesions. Moreover, maximum levels of hBD2 expression were revealed in the pustular lesions.34 In another study, different P. acnes strains were treated by keratinocyte cultures and an increase was noted in hBD2 messenger RNA expression by certain P. acnes isolates, while some isolates were shown not to effect hBD2 expression. Moreover, P. acnes-mediated hBD2 elevation was found to be inhibited by anti-TLR2 and anti-TLR4 antibodies. This finding suggests that the P. acnes-mediated AMP release by keratinocytes is dependent on TLR2 and TLR4.7 In a study investigating the levels of hBD1 and hBD2 expression in healthy human hair follicles and papular, pustular, and comedonal AV lesions and perilesional skin using immunohistochemical methods, Philpott36 detected strong hBD1 and hBD2 immunoreactivity in the whole suprabasal epidermis, hair follicles, and the pilosebaceous canal. In contrast, hBD1 and hBD2 expression was absent in the proximal follicle section progressing to apoptotic regression. In addition to marked hBD2 expression, partial hBD1 expression was also detected particularly in the lesional and perilesional epithelium of the pustular lesions.36
Medications used for AV treatment can also alter AMP expression. In the ex vivo lipopolysaccharide-induced inflammatory skin explant model, Poiraud et al.39 demonstrated increased hBD2 and psoriasin expression after zinc gluconate therapy but did not find any change in hBD4 expression. Harder et al.40 evaluated the stimulation of hBD1, hBD2, hBD3, and hBD4 gene expression in the keratinocytes and established that all-trans retinoic acid inhibited the pro-inflammatory cytokine-mediated expression of the hBD2, hBD3, and hBD4 genes, but did not alter the expression of the hBD1 gene. Borovaya et al.41 evaluated the effects of isotretinoin treatment in AV on the levels of AMP expression using RT-PCR. They measured the levels of AMP expression before isotretinoin therapy, during therapy, and after 6 months of continuous therapy. Compared to the control group, expression of cathelicidin, hBD2, lactoferrin, lysozyme, psoriasin, koebnerisin, and ribonuclease-7 increased in pre-treatment patients with AV, while the expression of α-defensin-1 decreased. Moreover, the expression of cathelicidin, hBD2, lactoferrin, psoriasin, and koebnerisin decreased during isotretinoin therapy, and the expression of cathelicidin and koebnerisin reached normal levels after 6 months of therapy. On the other hand, increased lysozyme and ribonuclease-7 expression was not affected by isotretinoin therapy and the authors suggested that lysozyme and ribonuclease-7 have stronger antimicrobial than pro-inflammatory activity. The authors concluded that the expression levels of several AMPs can be altered in the presence of acne.41
Cathelicidins belong to the AMP family.8 Wang et al.35 reported that the first AMP described in reptiles was cathelicidin, and an AMP called cathelicidin-BF exists in the venom of Bungarus fasciatus snakes. In that study, experimental administration of cathelicidin-BF in topical gel form on rat tissues provided strong antibacterial and anti-inflammatory efficacy against P. acnes. Thus, the researchers suggested that the cathelicidins may represent an alternative therapeutic option for AV.35
In a previous study we evaluated the levels of hBD1 and cathelicidin expression in different AV lesions such as comedonal, papular, pustular, and nodular lesions, according to different regions of the lesion including the epidermis, site of inflammation, dermis, and skin appendages. We observed that in the epidermis, hBD1 expression was elevated in the comedonal lesions compared to the nodular lesions. On the other hand, cathelicidin expression was lower in the inflammation area in comedonal lesions.1
In addition to their antimicrobial effects, AMPs also show anti-inflammatory effects. McInturff et al.42 demonstrated that granulysin-derived peptides, as well as showing antimicrobial effects, exert anti-inflammatory activity by inhibiting P. acnes-induced cytokine release.
CONCLUSIONS
In conclusion, the discovery of TLRs and AMPs introduced a new perspective to the investigations of the innate immune system. However, there is still a lot to elucidate about the biology of TLRs and AMPs. TLRs and AMPs play crucial roles in AV pathogenesis and extensive studies on this matter will increase the understanding of AV pathogenesis. Different therapeutic options targeting the regulation of the release of TLRs and AMPs may provide successful outcomes in AV treatment.