Abstract
Psoriasis is a chronic inflammatory skin condition that impacts patients’ quality of life and has large economic consequences. While current biologics are remarkable for their efficacy and safety, opportunities for improvement exist due to their rare side effects, fading efficacy, method of delivery, and expense. Biologics such as bimekizumab offer high likelihood of clearance, while oral options (e.g., deucravacitinib) allow patients to avoid injections and achieve efficacies similar to adalimumab or ustekinumab. As a result, there is limited room for the development of new biologics. Several oral therapies such as the oral monoclonal microbial EDP1815 have the potential to meet patient expectations for efficacy and convenient administration. However, emerging treatment regimens for plaque psoriasis will increasingly require a multimodal approach, addressing patient adherence, lifestyle choices, and awareness of the individual’s underlying pathophysiological processes. In this narrative review, the authors discuss recent advances in the development of biologic and oral small molecules for plaque psoriasis.
BACKGROUND: PSORIASIS STANDARD OF CARE
Disease Background and Overview of Treatment
Psoriasis is a chronic inflammatory skin condition that affects 2–3% of the population in the USA and has extensive psychosocial and economic consequences.1 Sustained inflammation leads to uncontrolled proliferation and differentiation of keratinocytes. Although psoriasis variants share a root pathophysiology, variations in pathways lead to variable treatment effectiveness.2 There are three categories of treatment for plaque psoriasis: topical medications, phototherapy, and systemic therapies. Topical therapies are the first-line treatment for localised psoriasis.3,4 Ultraviolet (UV) light can be used for localised and extensive psoriasis.3 Home phototherapy units can reduce cost and increase convenience. Nevertheless, the use of UV for moderate-to-severe psoriasis is declining in popularity, likely due to perceived high price, poor reimbursement, and the availability of highly effective, safe, and more convenient systemic treatments.5-7 The presence of moderate-to-severe disease often requires treatment with systemic therapies. Of the available systemic treatments, current professional dermatological association guidelines recommend biologics for the treatment of moderate-to-severe plaque psoriasis that is recalcitrant to local therapies.8
Systemic Therapy: Indications and General Options
Among biologic therapies, three classes are used for moderate-to-severe psoriasis: TNF-α inhibitors, IL-17 inhibitors, and IL-23 inhibitors.8
TNF-α inhibitors
The first generation of TNF-α inhibitors has potent initial efficacies. The most common method of reporting efficacy is the Psoriasis Area and Severity Index (PASI), a standard tool for scoring psoriasis severity. Following 12 weeks of use for infliximab or adalimumab, PASI90 (i.e., a 90% improvement from baseline) was achieved in approximately 50% of patients in treatment arms.9-11 PASI75 was achieved by 49% of patients treated with etanercept 50 mg twice a week at 12 weeks (p<0.0001). When combined with phototherapy, etanercept therapies have achieved a PASI90 in 58.1% over the same timeframe.12,13 These strong initial responses often fade. In the Danish DERMBIO registry study of 1,867 psoriasis treatment courses, 41.3% of these regimens were discontinued. The most common reason for discontinuation was the loss of efficacy (67.0%).14 TNF-α inhibitors are contraindicated in those with a strong family history of malignancy or chronic infection (e.g., tuberculosis, hepatitis B virus).3 As a result, there was an opportunity to develop novel therapies that averted some of the major class side effects, including the risk of serious infections (e.g., tuberculosis), malignancy, and major adverse cardiovascular events.15
TNF-α inhibitors also face competition from biosimilars. Recent adalimumab biosimilars include mjevita (adalimumab-atto), Cyltezo (adalimumab-adbm), Hyrimoz (adalimumab-adaz), Hadlima (adalimumab-bwwd), Abrilada (adalimumab-afzb), and Hulio (adalimumab-fkjp), with more on the way. Infliximab biosimilars include Inflectra (infliximab-dyyb), Renflexis (infliximab-abda), Ixifi (infliximab-qbtx), and Avsola (infliximab-axxq).16-19 The influx of competitive agents may lead to a decrease in cost.
IL-17/23 axis
The IL-17/IL-23 axis is central to psoriasis pathophysiology, and inhibitors of these cytokines are effective psoriasis treatments.3 The IL-17 inhibitor class consists of secukinumab, ixekizumab, and brodalumab.4,8 The IL-23 inhibitor class contains inhibitors to the p19 subunit of IL-23 (guselkumab, tildrakizumab, and risankizumab) and ustekinumab, which binds the p40 subunit of IL-23 that is also in IL-12.4 Many of these agents are both highly efficacious and generally well tolerated when used as treatments for moderate-to-severe plaque psoriasis. Ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab, and tildrakizumab have all entered clinical use for the treatment of moderate-to-severe plaque psoriasis.20 In a systematic review, ixekizumab appears to be the most effective short-term agent (i.e., greatest PASI75/90 over 12 weeks [p<0.00001/0.00001]). It is also the most likely to be associated with one or more adverse effects (AE) (p<0.00001). Ixekizumab 80 mg, risankizumab 150 mg, and brodalumab 210 mg have similar numbers needed to treat for PASI75 (1.18, 1.19, and 1.19, respectively).21
As novel therapies, the long-term AEs of the IL-17 and IL-23 classes are not fully known. Throughout recent years of clinical testing, they appear to be very safe. However, similarly to TNF-α inhibitors, efficacy can fade over time, perhaps due to neutralising antibodies.3,22 Unfortunately, these therapies can cost 50,000 USD or more annually.23 Though with several exceptions, most require administration by injection, which can be unfavourable for some patients.3,24
EMERGING THERAPIES
Biologics
IL-17 inhibitor: bimekizumab
Bimekizumab is an injectable IgG1 monoclonal antibody targeting IL-17A and IL-17F under development by UCB (Brussels, Belgium). Bimekizumab recently completed a direct comparison study against secukinumab in the BE RADIANT study, following its completion of comparison studies against adalimumab (BE SURE) and ustekinumab (BE VIVID). In this randomised, multicentre, double-blind study bimekizumab was compared to secukinumab, with the primary endpoint of PASI100 at 16 weeks.25 At 16 weeks, 61.7% of the bimekizumab arm achieved PASI100 versus 48.9% in the secukinumab arm. Analysis proved bimekizumab to be non-inferior, and, subsequently, superior to secukinumab (p<0.001 each). After 48 weeks, 67.0% of the bimekizumab arm achieved PASI100 compared with 46.2% in the secukinumab arm. However, it must be noted that there was a documented greater association with oral candidiasis in the bimekizumab arm.26
In the BE SURE trial, the most common side effects for bimekizumab were found to be nasopharyngitis (20.9%), oral candida infection (16.2%), and upper respiratory tract infection (9.0%). Throughout the trial, no serious AEs were reported in the treatment arm. Potential serious AEs included suicidal ideation or behaviour, inflammatory bowel disease, or major adverse cardiac events.
Previously, bimekizumab had undergone a Phase III, randomised, double blind, placebo-controlled trial (BE READY).27 The study enrolled 435 adults with moderate-to-severe plaque psoriasis. It consisted of an initial treatment period followed by a randomised withdrawal period lasting a combined 56 weeks. BE READY met its primary endpoints of PASI90 and an Investigator’s Global Assessment (IGA) response of clear or almost clear (IGA 0/1) compared to placebo after 16 weeks of treatment.27
In a previous Phase IIb study (BE ABLE 1) on bimekizumab’s safety, there was no association of bimekizumab with any dose-related safety risks or unexpected safety signals in a set of 250 patients. Treatment-emergent AEs occurred in 61% (126/208) of bimekizumab patients compared with 36% (15/42) of patients in the placebo arm. These AEs led to a study discontinuation rate of 4.8% (10/208) in the bimekizumab arm and 2.4% (1/42) in the placebo arm. The side effects leading to discontinuation included the diagnosis of colon cancer and, in a separate case, a large gastrointestinal polyp, which was deemed unrelated to the study treatment by the investigator.28
Inhibitory receptor agonist: CC-90006
CC-90006 is a subcutaneous injection programmed cell death protein-1 agonist antibody being developed by Celgene (Uxbridge, UK) for the treatment of mild-to-moderate plaque-type psoriasis. It recently completed Phase I testing in a multicentre, randomised, double-blind, placebo-controlled, multiple-dose study to evaluate drug safety, tolerability, pharmacokinetics, and pharmacodynamics in 34 participants with mild-to-moderate plaque-type psoriasis.29 No results are currently available for this or any other previous trials. Phase I testing was completed on April 26th 2019, but results have not yet been posted.29,30
Oral Small Molecules
JAK family
Filgotinib (GLPG0634)
Filgotinib (GLPG0634) is an oral, selective JAK1 inhibitor developed by Galapagos (Mechelen, Belgium) for use in adults with psoriatic arthritis (PsA), ulcerative colitis, and Crohn’s disease. Filgotinib was recently approved for the treatment of rheumatoid arthritis by the European Medicines Agency (EMA) and Pharmaceuticals and Medical Devices Agency (PMDA), with initial clinical use beginning in 2020. In the USA, under a collaborative agreement, Gilead (Foster City, California) sought U.S. Food and Drug Administration (FDA) approval of filgotinib for rheumatoid arthritis. In August 2020, they discontinued the pursuit for approval in the USA.31 Results from the previous Phase II EQUATOR trial,32 a randomised, placebo-controlled trial for moderate-to-severely active PsA, are available. Primary study endpoints were based on American College of Rheumatologists 20 (ACR20), a composite measure defined as the improvement in at least 20% of tender and swollen joints among additional criteria and while in a state of minimal disease activity. At 16 weeks, 80% (52/65) of patients in the filgotinib group achieved ACR20 compared to 33% (22/66) in the placebo group (p<0.0001).33 During the open-label extension portion of the study, which was extended until 52 weeks, 33.6% of patients in the filgotinib arm achieved minimal disease activity response and 55.0% achieved ACR50.34 The drug is currently advancing to the pivotal Phase III PENGUIN clinical trial programme to confirm the safety and efficacy.35
PF-06826647
PF-06826647 is an oral once daily tablet that acts through the inhibition of tyrosine kinase 2. It is being developed by Pfizer (New York City, New York, USA) for patients with moderate-to-severe plaque psoriasis. The drug is currently in a randomised, double-masked, parallel assignment, Phase II study of 179 participants to investigate its safety and efficacy for patients with moderate-to-severe plaque psoriasis.36 The study was nominally completed on 26th November 2020, with results currently undergoing quality control as of 3rd May 2021.36
In a previous Phase I trial of PF-06826647 investigating safety, there were no clinically relevant differences in physical exam or lab data between the 400 mg, 100 mg, and placebo arms.37 In the psoriasis cohort, the patients in the 400 mg treatment arm experienced AEs at a rate of 80.0% compared to 45.5% in the 100 mg arm and 50% in the placebo arm. All AEs were mild in severity, but were not individually identifed. No serious or severe AEs of clinical relevance were reported.37
BMS-986165 (deucravacitinib)
BMS-986165 (deucravacitinib) is an oral selective tyrosine kinase 2 inhibitor developed by Bristol Myers Squibb (Uxbridge, UK) for the treatment of patients with moderate-to-severe plaque psoriasis. In recently published data from the POETYK PSO-138 and PSO-239 Phase II trials, 58.7%/53.6% of patients receiving deucravacitinib achieved PASI75 as compared to 35.1%/40.2% receiving apremilast, and 12.7/9.4% in placebo groups at 16 weeks. Following 24 weeks of treatment, 69.0%/59.3% of patients reached PASI75 compared to 38.1%/37.8% receiving apremilast. Of the patients achieving PASI75 at 24 weeks, 82.5%/81.4% of patients in the PSO-1/PSO-2 trials maintained PASI75 response at 52 weeks. No serious or severe AEs were reported, including severe or opportunistic infections or thrombotic events. Among mild AEs, nasopharyngitis (5.7–17.9% versus 7.6% placebo) and sinusitis (0–7.6% versus 0% placebo) were the most reported complaints.37,40
Receptor antagonists
Nuclear receptor antagonist: BI 730357
BI 730357 is a film-coated tablet that acts as a pyrazinone retinoic acid receptor orphan receptor γ (RORγ) antagonist. It is a ligand-regulated transcriptional factor with diverse roles in cell proliferation and differentiation.41 It is being developed by Boehringer Ingelheim (Ingelheim am Rhein, Germany) for the treatment of moderate-to-severe plaque psoriasis. It is currently in a Phase II, interventional, randomised, double-blind, placebo-controlled long-term extension study of 180 participants to assess safety, tolerability, and efficacy in patients with moderate-to-severe plaque psoriasis.42 It is estimated to be completed on 28th February 2027.42 A previous, shorter-term Phase II study on safety, tolerability, and efficacy in 274 patients with moderate-to-severe plaque psoriasis was completed in June 2021, but no results are currently available.43
IL-2 receptor agonist: C-92252
CC-92252 is an IL-2 receptor agonist and regulatory T-lymphocyte stimulant currently being developed by the Celgene for the treatment of adults with psoriasis. It is now in Phase I of clinical development in a three-part interventional, randomised, parallel assignment study with quadruple masking.44 With 133 enrolled participants, the study aims to evaluate pharmaceutical safety, tolerability, pharmacokinetics, and pharmacodynamics of ascending doses of CC-92252 in both healthy adult subjects and adult subjects with psoriasis. It is estimated to be completed on 30th September 2022.44
Anti-inflammatory agents
µ-opioid antagonists (naltrexone)
Naltrexone is a µ-opioid antagonist taken orally and is under study for treatment of mild psoriasis by the Jinnah Postgraduate Medical Centre (JPMC; Karachi, Pakistan). The therapy has recently completed a Phase I clinical trial for the use of low-dose naltrexone in mild psoriasis in a tertiary care hospital in Karachi, Pakistan.45 This interventional, single group assignment trial with no masking of 42 participants was completed on 30th September 2019. Following 12 weeks of low-dose naltrexone, relevant markers of psoriasis severity decreased compared with patient baseline before treatment: PASI by 4.96 (p<0.001), Dermatology Life Quality Index (DLQI) by 6.32 (p<0.001), and Investigator’s Global Assessment and Body Surface Area (IGA*BSA) by 3.9 (p<0.001). The efficacy of the drug may be due to regulation of lymphocyte responses, reduced cytokine production, and decreased mast cell activity. Via reduction of pruritus, systemic naltrexone can act in an adjunctive role to control patient scratching behaviours and minimise physical trauma to the affected area. Low-dose naltrexone is generally considered safe, but mood and liver abnormalities have occasionally been reported at high doses.46,47
Cannabinoids
The endocannabinoid system maintains many aspects of skin homeostasis, such as proliferation, differentiation, and release of inflammatory mediators.48 Cannabinoids may aid in the management of cutaneous diseases. They can act directly via neuronal modulation of peripheral itch fibres. They can also act centrally on cannabinoid receptors with additional unspecified actions in the endocannabinoid system and cholinergic anti-inflammatory pathway.48,49 In refractory cases following standard therapies, cannabinoid formulations may be considered as an adjuvant therapy due to their ability to reduce symptoms of chronic pruritus in limited human studies.48,50
Brown University (Providence, Rhode Island, USA) has begun to investigate the impact of cannabis on pain and inflammation in patients with arthritis. In a Phase II, randomised, placebo-controlled, cross-over assignment, double-blind study.49 Brown University is exploring the effects of oral medium tetrahydrocannabinol or cannabidiol on pain symptomology and inflammatory markers in patients with rheumatoid or PsA. The study consists of 76 participants and has an estimated completion date of 31st July 2022.49
Oral microbials
EDP1815
EDP1815 is an oral tablet form of a monoclonal strain of Prevotella histicola, selected for its potent anti-inflammatory pharmacology. It is being developed by Evelo Biosciences (Cambridge, Massachusetts, USA). It is currently in Phase II of development for the treatment of mild-to-moderate plaque psoriasis.51
In a Phase II, multicentre, randomised, double-blind, placebo-controlled trial, EDP1815 is being investigated for its efficacy and safety in the treatment of 225 participants with mild-to-moderate plaque psoriasis. It aims to identify an optimal dose in these patients and is expected to be completed by 23rd December 2021.52
Previously, EDP1815 had been explored in a Phase I, randomised, double-blind, placebo-controlled, ascending dose study in healthy participants, patients with atopic dermatitis and patients with mild-to-moderate psoriasis.53 The study was completed on 31st October 2019. Results are compared to placebo and following a 56-day treatment regimen. In the atopic dermatitis cohorts, there was a 62% difference in Eczema Area and Severity Index (EASI) score (p=0.034) and 71% difference (p=0.019) in IGA*BSA. On Day 56, 10/16 patients showed improvements in EASI, with three of those ten patients achieving an EASI75 clinical response. In the psoriasis cohorts, EDP1815 limited the production of inflammatory-mediator cytokines, including IL-1, -6, and -8, and TNF. There was no statistical significance in the incidence of nausea, vomiting, diarrhoea, or abdominal pain between treatment and placebo arms. No serious side effects were reported.54
PSORIASIS PHARMACOLOGY IN BROADER CONTEXT
Compared to today’s standard of care (e.g., IL-17/IL-23 inhibitors) (Table 1), emerging agents (Table 2) do not show apparent advantages in terms of efficacy or safety. Emerging agents will likely need to demonstrate a benefit outside of clinical efficacy (e.g., cost or ease of use) to ensure clinical adoption. By comparison, the pharmacology of the future promises increased effectiveness and adherence through personalisation. Technological advances in tissue imaging, analysis, and proteogenomics will elucidate disease pathophysiology and help individualise treatment regimens.67,68 Recent discoveries in inflammatory marker and regulator systems (including pentraxin, receptor-acting protein kinases, and mixed-lineage kinase domain-like pseudokinase) offer potential targets for therapeutic intervention.69,70 As advances are made in the understanding of mRNA regulation in psoriasis, opportunities will arise for more accurate monitoring and efficacious intervention.69,71,72
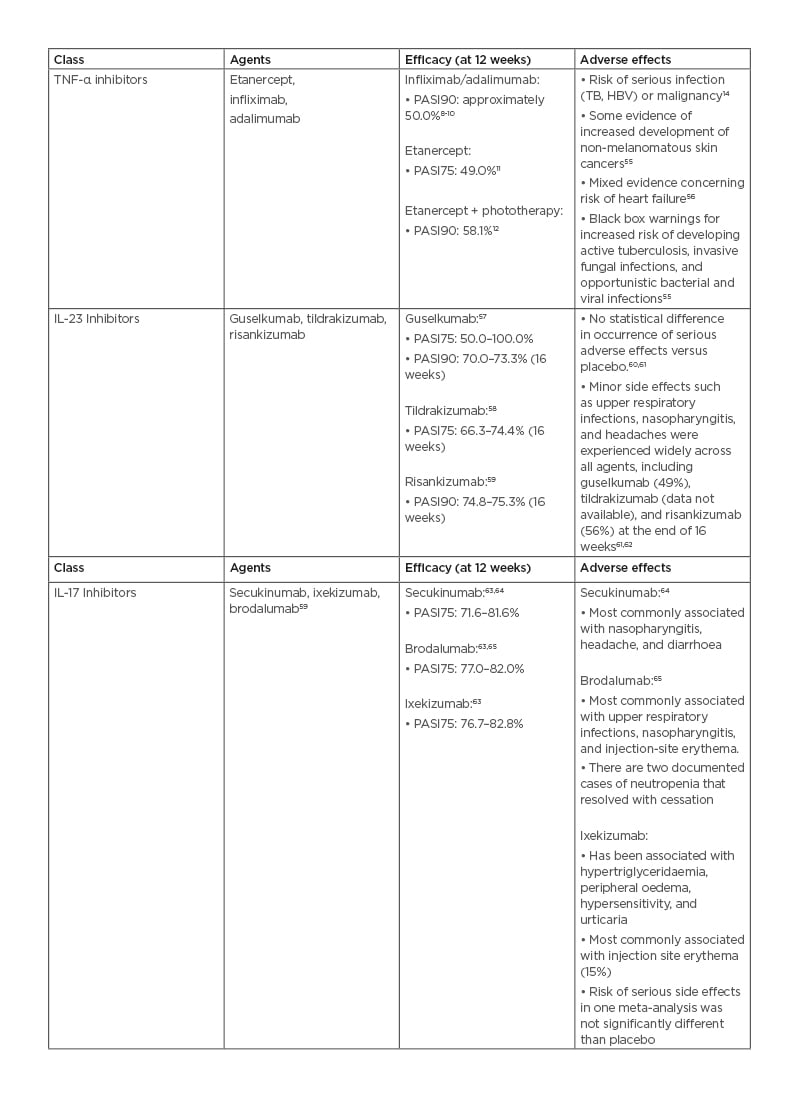
Table 1: Efficacy and adverse effects of current systemic plaque psoriasis therapies.
HBV: hepatitis B virus; PASI75: Psoriasis Area and Severity Index with 75% improvement from baseline; PASI90: Psoriasis Area and Severity Index with 90% improvement from baseline; TB: tuberculosis.
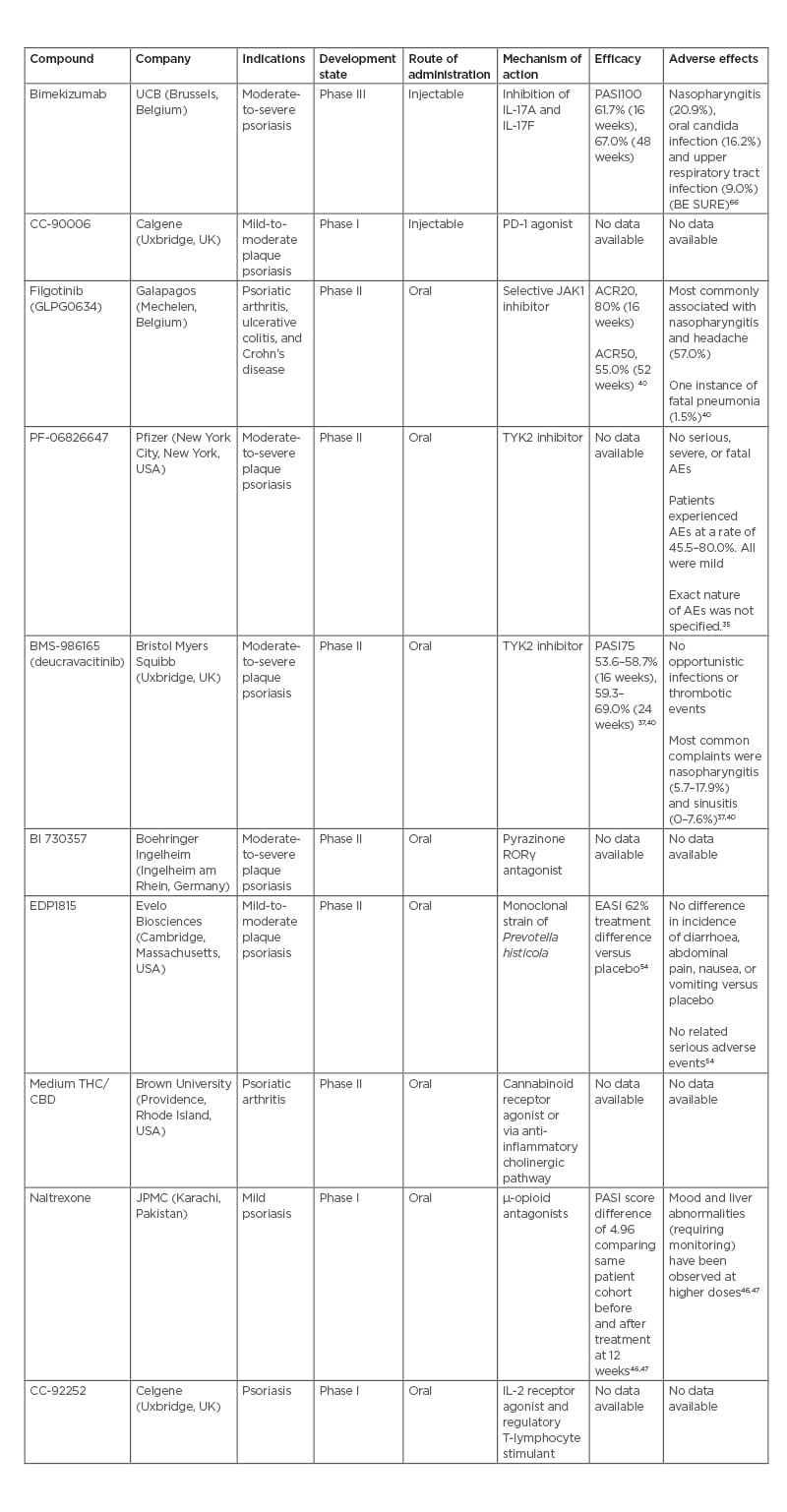
Table 2: Summary of discussed biologic and oral compounds.
ACR20: American College of Rheumatology score with 20% improvement; ACR50: American College of Rheumatology score with 50% improvement; CBD: cannabidiol; EASI: Eczema Area and Severity Index; PASI75: Psoriasis Area and Severity Index with 75% improvement from baseline; PASI100: Psoriasis Area and Severity Index with 100% improvement from baseline; PD-1: programmed cell death-1; RORγ: retinoic acid receptor-related orphan receptor γ; THC: tetrahydrocannabinol; TYK2: tyrosine kinase 2.
Exclusive discussion of pharmacology creates an incomplete picture of the advances made in psoriasis management. Understanding and combating poor medication adherence is essential to improving clinical outcomes. Currently, poor adherence not only leads to poor clinical outcomes, but also skews clinical trial data. It is important to note that adherence in clinical trials may be higher than in daily life when dosing is not monitored.73
Persistence rates vary significantly by agent and class. In a 2015 cross-sectional study, persistence rates were 19% for etanercept, 53% for adalimumab, and 71% for ustekinumab over 12 months (p<0.05).74 In a study of ustekinumab, which measured patient persistence rates over 6 months, ustekinumab showed a persistence rate of 81.4%; this is similar to the 80.6% and 87.4% persistence rates as seen in ixekizumab patients.75 The underlying reasons for these varying persistence rates remain complex. In one study, the most reported reason for discontinuing a psoriasis treatment regimen was ‘ineffective treatment’ (cited by 64.7% of patients). Poor dose-escalation schedule may impact these findings as patients may be pre-emptively discontinuing treatment due to perceived lack of efficacy. Alternatively, class efficacy may play a role. After switching to an IL-17 inhibitor, 45.7% of patients reported symptoms as “better,” while 26.5% reported no change in symptoms. Patients are more adherent to treatment regimens if their prescribing provider had specialty training in rheumatology.76 This finding may be due to greater adherence from patients with joint symptomology or the association of joint symptomatology with more severe skin findings. When appropriately treated, this patient population may experience greater improvement in quality of life than patients without rheumatological findings.
Medication adherence is associated with numerous factors outside of the treatment class used. Important negative predictors of adherence include a negative emotional state (i.e., presence of anxiety or depression) and unaddressed concerns regarding the disease, medication side effects, or potential for medication overuse. The evidence between demographic traits and adherence is more mixed, and definitive conclusions cannot be drawn.77,78 For patients with poor medication adherence, the three most common reasons for missing doses included forgetting (49%), feeling unwell (26%), and being too busy (15%).77 eHealth interventions (e.g., app notification services) have some effectiveness in using patient data to improve treatment adherence.73,79-81 Through clinical deployment, they may improve adherence through patient education and assistance in scheduling medication administration.
CONCLUSION
Overall, many patients with psoriasis have mild disease and could be adequately treated with a combination of topical treatments and phototherapy. For those requiring systemic treatment, current biologic therapies provide safety and efficacy but can be costly. The current market is competitive. Excellent efficacy rates are available through bimekizumab for those who seek the highest likelihood of clearance. Oral options are available (e.g., deucravacitinib) with efficacies similar to adalimumab or ustekinumab. Current clinical trials display no clear advantage in efficacy (Table 3). As a result, there is little room for the development of new biologics. Several oral therapies, including oral monoclonal microbials EDP1066, can meet patient expectations as favourable and effective treatments of plaque psoriasis. Emerging treatment regimens for plaque psoriasis will increasingly require a multimodal approach addressing patient adherence, lifestyle choices, and awareness of the individual’s underlying pathophysiological processes.
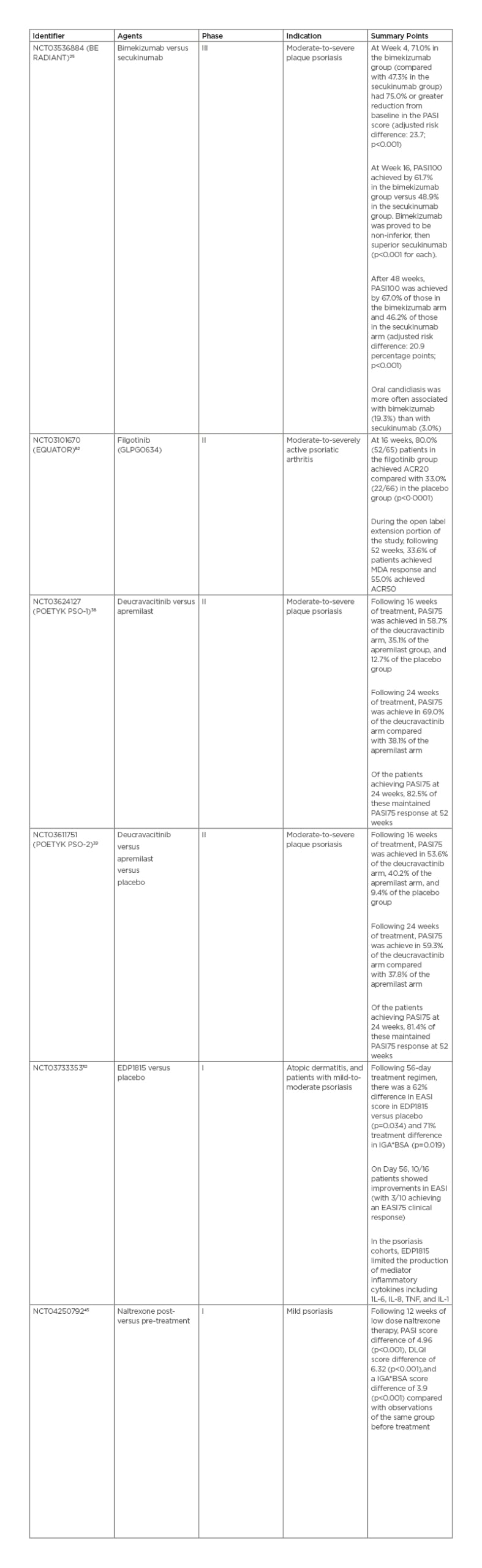
Table 3: Clinical trial summaries and associated efficacy results.
ACR20: American College of Rheumatology score with 20% improvement; ACR50: American College of Rheumatology score with 50% improvement; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; IGA*BSA: Investigator’s Global Assessment and Body Surface Area; MDA: minimal disease activity; PASI75: Psoriasis Area and Severity Index with 75% improvement from baseline; PASI100: Psoriasis Area and Severity Index with 100% improvement from baseline.







