Abstract
Serum sickness-like reaction (SSLR) is an acute inflammatory condition affecting children and adults characterised by the development of erythematous skin lesions and joint swelling with or without fever. Although these features resemble the ones seen in patients with classic serum sickness, the precise pathophysiology of SSLR remains unclear. It is considered that drugs, usually β-lactam antibiotics, and some infectious agents can trigger an immunologic reaction that leads to these clinical manifestations. This condition is usually under-recognised or mistakenly diagnosed as other conditions (e.g., urticaria, urticaria multiforme, reactive arthritis, erythema multiforme) and therefore infrequently reported. Until now, there was no standardised treatment for this condition and controversy regarding the use of antihistamines, nonsteroidal anti-inflammatory drugs, and oral corticosteroids remains. Most of the current literature on SSLR is based on occasional case reports series. The main objective of this manuscript is to offer an organised and updated review of the clinical features and current treatment options for paediatric SSLR, useful for physicians and other health professionals with interest in paediatrics and adverse drug reactions.
DEFINITION AND AETIOLOGY
Serum sickness-like reaction (SSLR) is an immunological condition characterised by skin rash and arthralgia, with or without fever. It can present in both adult and paediatric populations, although it is seen more often in children. Unlike classic serum sickness (SS), which also presents with similar clinical characteristics, SSLR is mainly triggered by drugs, mostly β-lactam antibiotics; however, vaccines and infectious agents have also been implicated in SSLR development.1 Classical SS was originally described at the beginning of the 20th century in patients who had received heterologous serum as antitoxins to treat diphtheria.2,3 Later, this condition was classified as a Type III immune hypersensitivity reaction, which is mediated by antigen-antibody complex formation. The accumulation of these complexes on small blood vessels from different tissues leads to complement activation and cytokine release, resulting in severe inflammation.4 Although the precise pathophysiology has not yet been elucidated, the mechanism by which drugs or other agents trigger SSLR appears to be different from the classic SS, because SSLR is not associated with antigen-antibody complex formation and the blood levels of complement are usually normal.5 Some theories consider the possibility that drugs, or their metabolites, may act similarly to haptens that bind plasma proteins and subsequently induce an abnormal immunologic response.6,7 Other studies have suggested that drug metabolites by themselves have a direct toxic effect on the lymphocyte affected patients.8,9 More recently, Zhang et al.10 reported that in children, antibiotics such as cefaclor may increase the intestinal mucosal permeability by damaging its integrity, which leads to the passing of antigens to the blood circulation favouring the development of SSLR. Likewise, other studies have demonstrated that the biotransformation of the parent drug in patients that develop SSLR induced by antibiotics, such as cefaclor, may be secondary to an inherited defect in the metabolism of reactive intermediates.8
Since its original description at the end of the last century, SSLR has been associated mostly with the use of antibiotics.11 Among these, β-lactams are the most commonly reported triggers, with one of the first drugs associated with SSLR in children being cefaclor.3,12 However, a great variety of other drugs have been reported to trigger SSLR: sulfonamide drugs, anticancer agents, anticonvulsants, anti-inflammatory agents, griseofulvin, metronidazole, bupropion, and more recently, biological agents such as rituximab, infliximab, and efalizumab, among others.13-16 Initial studies estimated that the incidence of SSLR in children caused by cefaclor was an estimated 0.4–0.5% of all antibiotic courses.12 Subsequent studies reported that SSLR represents 4.0% of all adverse drug reactions associated with amoxicillin.2 Other authors have reported that the risk of developing SSLR cefaclor is higher than amoxicillin.17 Overall, data regarding cross-reactivity among β-lactam antibiotics in children with SSLR are scarce, although it is considered that patients with SSLR attributable to cefaclor usually do not have to avoid other cephalosporins or penicillin because this reaction appears to be more compound-specific than class-specific.9
To the authors’ knowledge, there is no estimated incidence for SSLR caused by infections; although, infectious agents have also been reported as potential triggers of SSLR. To date, there is no literature available regarding studies that evaluate their role on its pathogenesis. In the past, vaccines were also associated with the development of SSLR; however, the current literature in paediatric patients is outdated and limited. A 1987 report by Milstien et al.18 described ten cases of paediatric patients that developed SSLR after exposure to Haemophilus influenzae Type B vaccination; nevertheless, the criteria used to diagnose SSLR are questionable as many of them did not consider joint involvement or associated skin rash.19 No further cases of SSLR associated to vaccines have been reported in children, but a a small number of case reports and series in adult populations have suggested an association between H1N1 influenza vaccination and SSLR.20,21
Currently, paediatric SSLR is considered an uncommon adverse drug reaction; however, its precise prevalence is unknown. This is mostly because of the lack of knowledge of some health professionals regarding this condition and its clinical presentation; therefore, SSLR is usually unrecognised or easily mistaken by other cutaneous entities such as urticaria, urticaria multiforme, erythema multiforme, infectious rashes, or other drug reactions.
CLINICAL FEATURES
Initial reports of patients with SSLR described presentation of skin rash associated with joint inflammation or arthralgia with or without a fever.22,23 The morphology of the skin rashes reported in the literature varies widely including morbilliform rash, urticarial and annular plaques with central clearing, or erythema multiforme-like lesions (erythematous annular converging plaques with purplish/dusky centre) (Figure 1A). It is also reported that, unlike acute urticaria, skin lesions in SSLR are not migratory, but are fixed. Once skin lesions develop, they stay in the same area until they resolve, and occasionally leave a bruise-like postinflammatory hyperpigmentation behind that may last for several days.6,24 The skin lesions usually start as small erythematous papules or plaques on the trunk and then enlarge and progressively spread to the rest of the body. With regard to facial impact, periorbital or lip swelling may present resembling angioedema; however, these patients do not develop tongue swelling or respiratory compromise as seen in other allergic reactions.
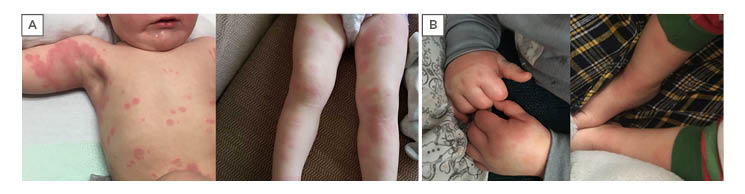
Figure 1.
- A) Erythematous papules and annular lesions, some of them with a polycyclic arrangement, central clearing and purplish discolouration.
- B) Painful inflammation of hands and feet (notice erythema and oedema overlaying the joints.
Unlike classical urticaria, itchiness in SSLR is mild or nonexistent; however, skin soreness or burning sensation may present instead. Given the common presence of knowledge gaps in dermatology, other health professionals often misdiagnose SSLR with erythema multiforme because skin lesions in SSLR may present with a violaceous centre that simulate target lesions. Unlike erythema multiforme, SSLR lesions do not have three rings (typical target lesion), do not blister, and have no involvement of mucous membranes.3
Alongside skin rash, the presence of joint involvement, characterised by joint swelling and arthralgia, is also necessary to make the diagnosis of SSLR. Joints are usually affected bilaterally but may present on only one side. Joints in the hands and feet are the most commonly involved, followed by knees, and then elbows. Purple discolouration and oedema may also be seen on the skin overlaying the joints5 (Figure 1B).Although oedema of the hands and feet (without arthralgia) could also present in other conditions such as urticaria multiforme and urticaria, joint inflammation in children with SSLR lasts for several days and the associated pain is disabling: parents usually report that children with SSLR avoid walking or move abnormally.9,23
Unlike classic SS, fever in children with SSLR may not be present. Concomitant fever ranges from 30% to 75% in these patients.6,23,25 Likewise, paediatric SSLR seldom presents with lymphadenopathy or systemic involvement; however, malaise and irritability lasting several days or weeks even after the rash has resolved has been reported.26
The development of skin rash and arthralgia in children with SSLR can present several days after exposure of the trigger, ranging from a couple of days up to several weeks. In the case of SSLR induced by antibiotics, the clinical features typically appear after the course has been completed (approximately 7–10 days).7,23 There are several studies that have confirmed up to 80% of paediatric patients with SSLR exposed to the same drug at least once show no previous reaction. There are no studies which have established the recurrence rate for SSLR in these patients; although, evidence has suggested those who had experienced an event of SSLR and were then re-exposed to the same medication presented with early and more severe symptoms than the previous reaction.3,9,17 As it stands, there is a need for more robustly conducted studies to determine the risk of recurrence in these patients.
Finally, formal diagnostic criteria and scales for the severity of SSLR in children are lacking. Thus, a formal evidence-based consensus is needed in addition to well-controlled prospective studies to develop these. A summary of a series of cases of paediatric SSLR are shown in Table 1.
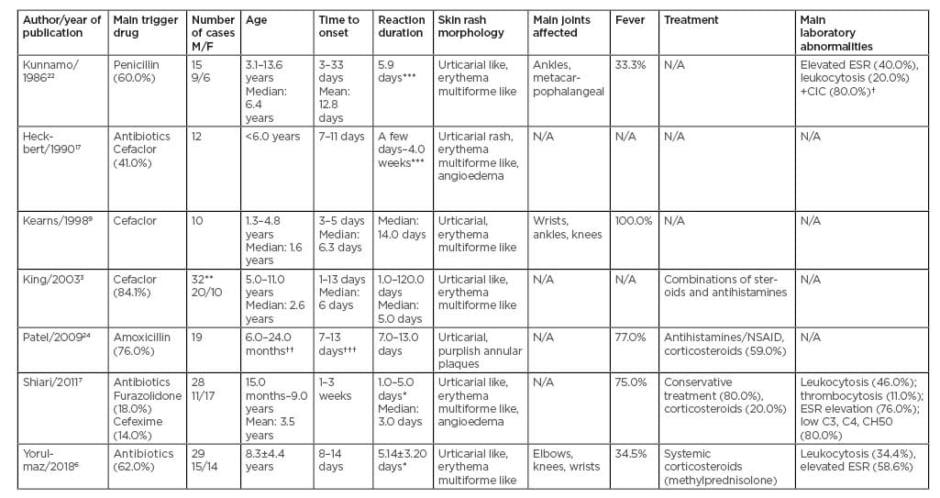
Table 1: Summary of series of cases of paediatric serum sickness-like reaction.
CIC: circulating immune complexes; ESR: erythrocyte sedimentation rate; M/F: male/female; NSAID: non-steroidal anti-inflammatory drug; N/A: data not available.
*Duration of hospital stay. ** In this study 44 patients were diagnosed with serum sickness-like reaction, but the data only refers to 32 patients with serum sickness-like reaction induced by cefaclor. *** Duration of joint symptoms.
† positive CIC. †† 59% of patients were in this range of age. ††† 53% of patients were in this range of days.
INVESTIGATIONS
Currently, the diagnosis of SSLR is primarily based on history and clinical features because the laboratory profile is usually nonspecific and in some cases seems contradictory. Shiari et al.7 reported a series of cases of 29 children with SSLR, of whom 46.0% showed leukocytosis on the complete blood test, 76.0% had high erythrocyte sedimentation rate, and 83.3% (20/24) had low levels of complement (C3, C4, and CH50); however, in other cases, levels of complement have been reported as normal or slightly elevated. A previous study performed in children with arthritis attributable to SSLR showed the presence of circulating immune complexes; however, no other study has replicated the findings.23 More recently, Yorulmaz et al.6 reported that, in addition to leukocytosis and elevated erythrocyte sedimentation rate, some patients presented with mild proteinuria or haematuria. Other studies also reported abnormal liver function tests and elevated creatinine.2 Skin testing and radioallergosorbent test were usually negative because SSLR does not appear to be an IgE-mediated reaction. Other in vitro tests, such as the lymphocyte toxicity assay, were used to evaluate the toxic effect of specific drugs on T cells from patients with adverse drug reactions, including SSLR. This test is not currently validated for the diagnosis of SSLR because the lack of a gold standard test means its predictive value remains difficult to define. Several studies, however, have demonstrated that T cells from SSLR patients have a higher sensitivity to specific medications compared to T cells from healthy controls.9,27
Skin biopsies are rare in children with SSLR because of their invasive nature; however, sometimes the histological features could help rule out other pathologies that share clinical features similar to SSLR. Some of these differential diagnoses include rheumatic fever, urticarial vasculitis, systemic lupus erythematosus, drug-induced lupus, Still’s disease, and Henoch-Schönlein purpura, among others.2,24 Histopathology is characterised by perivascular and mid-dermal inflammatory infiltrate with admixed neutrophils, eosinophils, and lymphocytes, usually without leukocytoclastic vasculitis.16,28
PROGNOSIS AND TREATMENT
Although SSLR is usually a self-limiting condition with no sequelae, complete resolution of the symptoms can take several days and even weeks. Additional to the immediate withdrawal of the causal drug when this is the case, medical treatment may be necessary. The latter focusses on the elimination and/or improvement of the symptoms and reduction of the disease course. Antihistamines and nonsteroidal anti-inflammatory drugs are used to control joint pain and itchiness. When symptoms are more severe and prolonged, the use of a short course of oral corticosteroids such as prednisone (0.5–1.0 mg/kg/d for 3–5 days) or intravenous methylprednisolone (10.0 mg/kg/d for 3 days) is recommended.1,6 Until now, there are no current guidelines for medical treatment of children with SSLR because there are no studies evaluating the effectiveness and safety of these therapies, so dosage and length of treatment are usually based on the severity of the symptoms and the experience of the healthcare professionals.5 The prognosis of children with SSLR is typically favourable because they have a mean recovery time of 5–7 days with no evidence of sequalae, even if they do not receive any treatment. Moreover, as previously stated, the rate of recurrence in children with SSLR is unknown but suspected to be high; therefore, avoidance of the trigger drug is highly recommended to prevent severe recurrences. Additionally, classical desensitisation does not appear to have a role in patients with SSLR because protocols for desensitisation were designed to treat Type 1 (IgE-mediated) mast cell reactions. There is no current evidence supporting this management in non-IgE-mediated and non-immune-mediated processes.29
CONCLUSION
SSLR in children is an immune reaction characterised by the development of skin rash and arthralgia with or without fever, and it is mainly associated with antibiotics from the β-lactam class. Skin lesions are characterised by fixed erythematous and oedematous patches/plaques and annular lesions with central clearing and/or purplish discolouration. Joint inflammation more frequently affects wrists and ankles bilaterally (hands and feet), but other joints can also be affected. Symptoms of SSLR usually develop several days after exposure to the trigger drug. Patients may have been exposed to the same drug previously without any complications. Although the prognosis of patients with SSLR is usually good, in some patients, the resolution of the symptoms may take several weeks. Laboratory studies are usually nonspecific; however, they may be useful to rule out other conditions. Treatment with antihistamines, nonsteroidal anti-inflammatory drugs, and/or systemic corticosteroids may reduce the recovery time and improve the symptoms but the use of these medications remains controversial and avoidance of the trigger drug is recommended. Further prospective and well-organised studies are needed to create more accurate diagnostic criteria which will help clinicians to better recognise the condition and establish a safe and effective treatment to reduce the morbidity associated with SSLR in children.







