Abstract
This paper describes an unreported case of a rifaximin-induced phototoxic reaction in an otherwise healthy 24-year-old female (skin type: V). The patient developed malaise, chills, and facial swelling with accompanying redness and itching that began within a day of initiating treatment with rifaximin (200 mg twice daily), and progressively increased over the next 3–4 days. The patient revealed that they had been lying in the sun for hours due to the chills they were experiencing. Over the next 10 days they developed an exaggerated, acute, sunburn-like phototoxic reaction, with blistering over the exposed skin. A skin biopsy showed no evidence of vasculopathy, endothelial damage, or extravasation of red blood cells. The patient was treated successfully with oral prednisolone (30 mg per day for a week), topical mometasone furoate (0.1%) cream applied twice daily, levocetirizine (5 mg per day taken orally), zinc oxide (20.0%) cream applied every 3 hours during daytime, and strict sun avoidance. The possible pathomechanism of rifaximin-induced sunburn is also discussed here.
Key Points
1. This case describes a 24-year-old female who developed a rifaximin-induced phototoxic reaction, with symptoms including malaise, chills, and progressive facial swelling with redness and itching, followed by an exaggerated, acute, sunburn-like phototoxic reaction after sun exposure.2. Treatment with oral prednisolone, topical mometasone furoate cream, levocetirizine, zinc oxide, and strict sun avoidance was successful in improving the phototoxic reaction.
3. While the pathomechanism of rifaximin-induced acute sunburn is not yet understood, dermatologists must remain vigilant as rifaximin is increasingly prescribed.
INTRODUCTION
Drug-induced photosensitivity is an adverse cutaneous reaction caused by a simultaneous exposure to potentially photosensitising drugs (via topical application or parenteral administration) and ultraviolet (UV) radiation or visible light. Clinically, drug-induced photosensitivity manifests as either a photoallergic drug reaction or a phototoxic drug reaction.1,2 In a photoallergic drug reaction, the photosensitising drug in the skin absorbs light photons to form a photoproduct, which binds to a soluble or membrane-bound protein to form an antigen. This reaction is immune-mediated and develops in only a small number of exposed individuals, depending on the presence of immunologic reactivity from previous sensitisation. The phototoxic drug reaction is non-immune-mediated and occurs in all individuals. Clinically, it is essentially an exaggerated sunburn response characterised by sharply demarcated painful erythema, oedema, and blistering over the exposed skin. The potentially photosensitising systemic drugs include: tetracyclines, sulfonamides, fluoroquinolones, thiazides, sulfonylurea antidiabetics, non-steroidal anti-inflammatory drugs, psoralens, pirfenidone, efavirenz, and taxanes.2-6 The chemicals used in topical antiseptic agents, fragrances, tanning lotions, sunscreens, and various cosmetics are other common causes of photosensitivity.7 This paper reports a rare case of an acute phototoxic drug reaction from rifaximin and discusses the possible pathomechanism involved.
CASE REPORT
A 24-year-old female was hospitalised with multiple erythematous acrofacial photodermatitis, which was causing pain and a burning sensation. Historically, they had previously developed gastroenteritis and were treated with rabeprazole (20 mg per day), levosulpiride (75 mg per day), and rifaximin (200 mg twice daily). As their nausea improved, they stopped taking rabeprazole plus levosulpiride after their second dose, and continued rifaximin in the prescribed doses. The patient developed malaise, fever (not documented), chills, and facial swelling, with accompanying redness and itching that began within a day of initiating the treatment and was progressively increasing over the next 3–4 days. The patient also revealed that they had been lying in the sun for hours due to the chills they were experiencing.
Over the next 10–12 days, the facial rash increased, and similar lesions associated with pain and intense burning had also appeared over the dorsum of their hands and feet. The patient related that the onset of their abdominal symptoms was incidental. Their personal and family histories were unremarkable, they were not taking any medication, and they had no previous history suggestive of any drug sensitivity, lupus, or other photosensitive disorder. A cutaneous examination (Figure 1) showed mild oedema and intense diffuse erythema, marked by well-demarcated darkening, cracking, and peeling, which varied in intensity and was limited to the directly sun-exposed skin over the forehead, malar area, lower lip, chin, presternal skin, the dorsa of hands, around ankles, and the borders of the feet and adjoining soles, with blistering in places. The mucosae were normal, except for the lower lip, which displayed erythema, fissuring, and a small amount of crusting. The hair, nails, palms, and other systemic examinations were normal. Due to the possibility of a rifaximin-induced acute phototoxic reaction, all of the previous treatments were stopped. The haemogram, hepatorenal and thyroid function tests, antinuclear antibody titres, 24-hour urinary proteins, chest X-ray, and urinalysis showed no abnormality. Histology from the erythematous skin lesion over the ankle showed hyperkeratosis, mild spongiosis, and a perivascular inflammatory cell infiltrate composed of lymphocytes and occasional neutrophils in the upper dermis (Figure 2). There was no evidence of vasculopathy, endothelial damage, or extravasation of red blood cells. Direct immunofluorescence was not performed because of financial constraints.
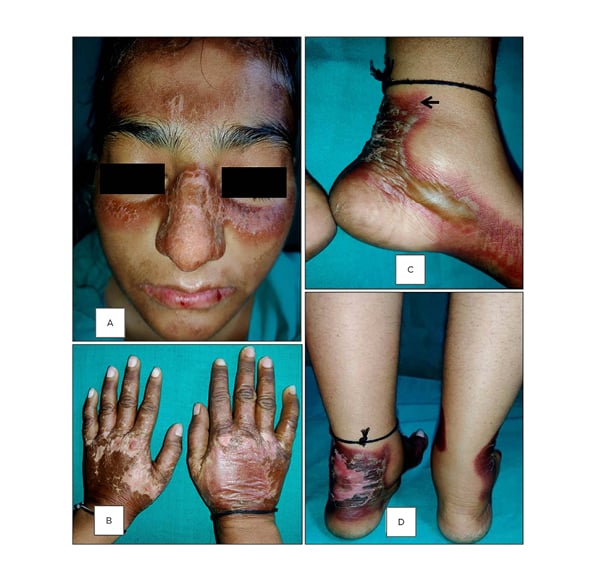
Figure 1: Photographs of the patient’s acute sunburns, characterised by diffuse erythema.
This is marked by the darkening, cracking, and desquamation of the skin in variable intensity, involving the sun-exposed sites: A) the forehead, malar area, lower lip, and chin; B) the dorsa of the hands; C) the borders of feet and adjoining sole; and D) around the ankles. Similar lesions were present over the toes and presternal skin. Note the sharp demarcation of all of these lesions. The blistering around the ankle in C) suggests early severe acute phase, and the arrow in this image indicates the site of biopsy.
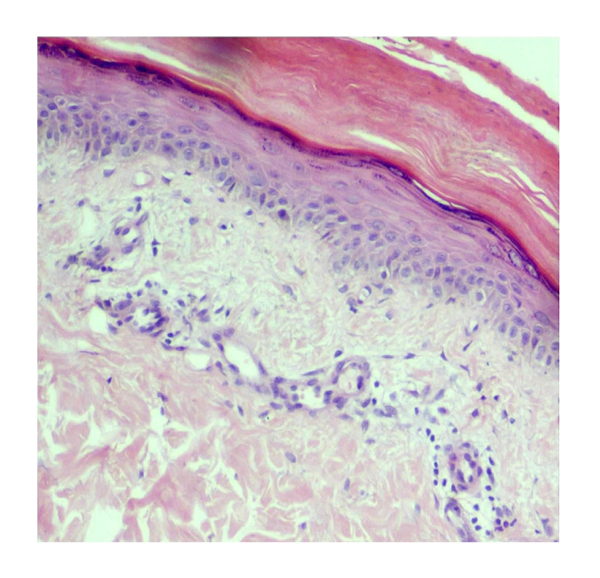
Figure 2: A light microscope image of the erythematous skin lesion over the ankle of the patient using a haematoxylin and eosin stain (x40).
Histology showed hyperkeratosis, mild spongiosis, and a perivascular inflammatory cell infiltrate in the upper dermis composed of predominantly lymphocytes and occasional neutrophils. The absence of vasculopathy, endothelial damage or swelling, and the extravasation of red blood cells is notable.
The patient was treated with oral prednisolone (30 mg per day for a week), topical mometasone furoate (0.1%) cream applied twice daily, levocetirizine (5 mg per day taken orally), zinc oxide (20.0%) cream every 3 hours during daytime, and strict sun avoidance. A review carried out 4 weeks after hospital discharge showed that all lesions had resolved with hypopigmentation. The patient was advised to avoid sun exposure and to topically apply physical sunscreen (sun protection factor: 19) containing 7.5% micronised zinc oxide (Sunstop-19™ [Ajanta Pharma Ltd, Mumbai, India]) every 4 hours on all days. At a recent visit, approximately 6−7 months after their last review, the patient revealed that they did not continue treatment beyond one month or so. The patient showed no residual dyspigmentation or recurrence of photosensitivity, and they did not consent for phototesting or photopatch testing.
DISCUSSION
Rifaximin is a semisynthetic, structural analogue of rifamycin and a non-systemic, site-specific gastrointestinal antibiotic. It reduces bacterial virulence and translocation, has anti-inflammatory properties, positively modulates the gut microbial flora, and induces eubiotic changes in the intestinal ecosystem. Rifaximin is effective for the treatment of travellers’ diarrhoea, caused by Escherichia coli, and other gastrointestinal infective conditions, such as Clostridium difficile colitis. Additionally, it is useful for treating diarrhoea-predominant irritable bowel syndrome and reduces the risk of overt hepatic encephalopathy recurrence. Rifaximin is poorly absorbed after oral administration, does not significantly pass the gastrointestinal wall, and undergoes metabolism with >90.00% excretion in the faeces, negligible excretion of unchanged drug in urine, and <0.01% distribution in other tissues.8-10
Hypersensitivity reactions, such as anaphylaxis, angioedema, urticaria, pruritus, flushing, fatigue, fever, chills, malaise, headache, gastrointestinal upset (abdominal pain, constipation), and respiratory tract infection, were common mild adverse effects in <2% of patients taking rifaximin in clinical trials and post-marketing reports.9 While pruritus, rash, and cellulitis were common dermatological adverse effects in 1–10% of cases, sunburn was rare, occurring in only 0.1–1.0% of cases.9 Although the authors could not perform a drug rechallenge, rifaximin could definitely be the cause of the patient’s pruritus, and facial erythema and swelling, and could be associated with malaise, chills, facial oedema, and the rash that had developed within a day. However, the authors suggest that more progressive severe acute sunburn occurred as a result of undue sun exposure (temporal correlation). This seems most plausible as, clinically, the phototoxic drug reaction usually starts within few hours and reaches a peak between several hours to a few days after exposure, eventually forming hard, blackish, rough, brittle, cracked, and desquamating plaques, as was noted in this case.1 Once treated, resolution occurred without recurrences after drug discontinuation (dechallenge), as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment system scale.
The possibility of subacute cutaneous lupus erythematosus (SCLE) arising because of rabeprazole plus levosulpiride, which is known to aggravate pre-existing lupus or trigger new onset SCLE, could be excluded due to the fact that only two doses of the drug were ingested by the patient and there was no clinical or laboratory evidence of pre-existing lupus erythematosus. Moreover, it usually takes weeks to many months for drug induced-SCLE to develop after the initiation of the offending medication.11,12
The pathomechanism of rifaximin-induced sunburn is poorly understood due to the paucity of cases. In general, UVB rays (290–320 nm) can induce photosensitisation more efficiently than UVA rays (340–400 nm) in the absence of an exogenous photosensitiser. However, acute phototoxicity injury has been reported with exposure to UVA rays, even in sub-erythematous amounts.1 Although UVA rays are more melanogenic than erythrogenic to produce acute erythema, the action spectrum for exogenous photosensitisers usually includes UVA rays that penetrate further than UVB, but less than visible light. However, the severity of photosensitivity is also dictated by factors such as gastrointestinal absorption, distribution and metabolism of the offending drug, skin colour, stratum corneum thickness in the host, and the amount of UV radiation and/or sun exposure.1 Although rifaximin is primarily a site-specific gastrointestinal antibiotic, in pharmacokinetic studies, 18% of the radioactivity in plasma reflects systemic absorption and the potential for its accumulation, which can cause adverse effects in skin.9
The interaction of the photosensitising molecule, the non-ionising radiation, and the affected skin occurs either from oxygen-dependent or photodynamic reactions, as in porphyrin phototoxicity, or oxygen independent or non-photodynamic reactions, as in psoralen phototoxicity. Photo-induced cellular damage results from phototoxicity to either cellular DNA or RNA, as with psoralens, or mitochondria, as with tetracycline.1 As the systemic photosensitising molecules reach the skin via the vasculature, the inflammatory changes remain limited to the dermis without keratinocyte apoptosis, whereas the dermal blood vessels are damaged in porphyrin-induced acute phototoxic reactions. However, any association of rifaximin-induced sunburn with the metabolism of porphyrins seems unlikely, which appears plausible in the absence of any significant epidermal or vascular changes noted at microscopic level, but dermal perivascular inflammation in this case corroborates the former. Overall, the exact pathomechanism of rifaximin-induced acute sunburn remains conjectural and open to debate. Nevertheless, dermatologists must remain vigilant of this rare cause of severe phototoxicity, as additional cases may be seen in the future with the increasing prescription of rifaximin.
LIMITATIONS
The cause of gastroenteritis in this patient could not be ascertained. However, drug rechallenge, phototesting and photopatch testing, direct immunofluorescence, and immunological tests for extractable nuclear antigens or rheumatoid factor were not performed because of a lack of consent and financial constraints.






