Abstract
Morphoea is a chronic inflammatory disease of the skin and underlying tissues, characterised by fibrosis of the skin and subcutaneous tissue without systemic involvement. Radiation-induced morphoea is a rare, often unrecognised, chronically progressive form of radiation-associated localised scleroderma of the skin, infrequently progressing to systemic scleroderma. Systemic scleroderma is characterised by widespread tissue fibrosis along with systemic features, leading to an increased risk of malignancy compared to the general population. The authors present the case of a 67-year-old female, a previously diagnosed case of psoriasis developing squamous cell carcinoma on the ring finger with axillary nodal metastasis, who underwent radiotherapy, and subsequently developed symptoms of systemic sclerosis. This case is unique, as there are nominal reports on generalised morphoea converting to systemic sclerosis post-radiotherapy, along with exaggerated thickening of the skin over the side of radiotherapy. Thus, the authors report this rare side effect of radiotherapy. Treating physicians should always be vigilant in identifying these side effects after radiotherapy to prevent long-term sequelae of the disease, and minimise their impact on the patient’s quality of life.
Key Points
1. The article highlights the crucial role of vigilance in diagnosing rare conditions that may arise following radiotherapy in patients with cancer, to detect any post-irradiation complications and worsening of connective tissue disorders such as systemic sclerosis (SSc).
2. The article reports on a rare case of SSc that occurred after radiotherapy. This case is distinctive as it presented a diagnostic dilemma between post-irradiation morphoea and SSc, initially due to the absence of systemic complaints, and later on, increased cutaneous involvement with systemic symptoms. This dilemma suggested the possibility of de novo SSc secondary to radiotherapy, or the conversion of radiation-induced morphoea to SSc.
3. The key message to take away is to consider SSc and post-radiation morphoea as potential differential diagnoses in patients who present with cutaneous side effects of radiation. Dermatologists should make a timely diagnosis to start treatment early, and prevent the progression of the disease
INTRODUCTION
Morphoea causes skin and subcutaneous tissue fibrosis, and can sometimes involve the fascia, muscle, or bone. Sclerosis usually begins as an erythematous or violaceous plaque or patch that becomes indurated. The absence of internal organ involvement, sclerodactyly, nail fold capillary changes, and Raynaud’s phenomenon distinguishes morphoea from systemic sclerosis (SSc).1 The exact cause of morphoea is unknown. Several triggers have been identified, including traumatic injury, infection, chemicals, and radiation exposure. Unlike idiopathic morphoea, which has a reported incidence rate of 2.7 per 100,000 persons, the incidence of post-irradiation morphoea is estimated to be as high as one in 500 patients.1 Patients who receive focal radiotherapy are more likely to develop morphoea; most of these have lesions restricted to the radiation field and nearby tissue.1 A study conducted at Johns Hopkins University, Baltimore, Maryland, USA, and Pittsburgh University, Pennsylvania, USA, to see the impact of radiation therapy in patients with breast cancer with systemic scleroderma, concluded that local tissue fibrosis is not inevitable in SSc, occurring in 50% of cases without any evidence of lung or generalised skin fibrosis flare.2
Psoriasis, a chronic inflammatory disorder, also increases the risk of developing non-melanoma skin cancer, especially in patients treated with psolaren and ultraviolet light A, methotrexate, or biologicals.
The authors’ case is unusual as, secondary to radiotherapy given for squamous cell carcinoma (SCC) with axillary nodal metastasis, the patient developed localised morphoea that progressed to generalised morphoea, also affecting radiation-unexposed sites, and later on also developed systemic manifestations of SSc within 24 months of radiotherapy.
CASE REPORT
A 67-year-old female, a previously diagnosed case of psoriasis for the last 8 years, was being treated with methotrexate 10 mg/week. The patient was non-compliant, with exacerbation of psoriasis during winter. In July 2019, they presented with a raised lesion, having central depression on the right hand’s lateral aspect of the ring finger. The patient was further advised for an MRI of the hand, which was suggestive of an altered signal intensity lesion, necessitating further evaluation to rule out cancer. Ultrasonography of the axilla was performed pre-operatively to evaluate nodal metastasis, revealing the presence of pathological lymph nodes (approximately 23 mm in short axis with cortical thickness of 7 mm, and loss of fatty hila, which were suggestive of malignant node by size and morphologic criteria).
Disarticulation of the ring finger along with axillary lymph node dissection was done, as the excisional biopsy from the finger revealed SCC with perineural invasion. One of the nine lymph nodes sampled was positive for metastasis. The patient’s case was discussed by a multidisciplinary tumour board, and adjuvant radiotherapy was planned in September 2019. Over the course of 5 weeks, the right axillae, supra, and infraclavicular fossa were irradiated with a dose of 50.4 Gy/28 fractions at 1.8 Gy/fraction. During ongoing sessions of radiotherapy, the patient developed an acute skin radiation reaction of Grade 2, which eventually subsided. The last dose of radiation was given in October 2019. Thereafter, the patient was asymptomatic for 1 year, after which they developed itching over the skin lesion, and presented to the authors’ outpatient department with a complaint of itching on the indurated plaque that developed over the trunk after almost 1 year of undergoing radiotherapy.
Cutaneous examination revealed well-defined, hyperpigmented, indurated plaque covering two-thirds of the back, involving the medial region of both breasts near the sternum, sparing the nipple-areolar complex, and extending to the upper abdomen, with depigmentation over the accessible sites due to severe itching (Figure 1A and 1B).
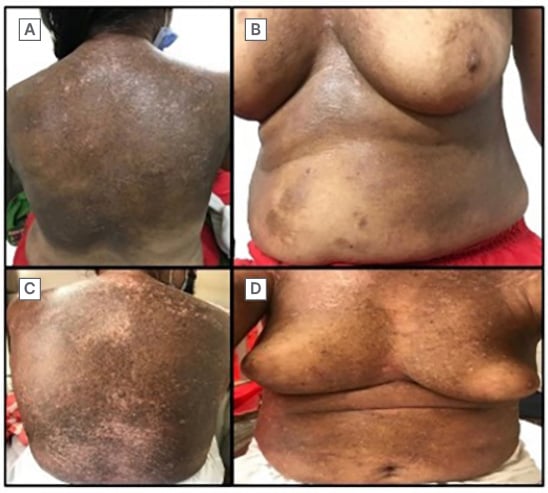
Figure 1: Clinical images comparing the area of involvement in both visits.
A) Well-defined, hyperpigmented indurated plaque with depigmented excoriations over the back. B) Well-defined, hyperpigmented patch over the sternum, also involving the medial areas of the breast and sparing the nipple-areolar complex. C) Increased area of involvement over the back compared with A.
D) Reduction in the bulkiness of the breast secondary to increased induration.
The skin biopsy from the back revealed morphoea. The antinuclear antibody (ANA) titre was 1:320. Additional testing for ANA subtypes was negative for anti-topoisomerase antibodies, anti-Smith antibodies, anti-centromere antibodies, anti-double-stranded DNA antibodies, anti-Sjögren’s-syndrome-related antigen A autoantibodies, anti-Sjögren’s antibodies, and anti-U1 ribonucleoprotein (RNP). The patient was started on 10 mg methylprednisolone daily and topical 0.1% tacrolimus ointment, and was again lost to follow-up for the next 6 months.
During the 6 months, the patient developed Raynaud’s phenomenon, gastroesophageal reflux disease, exertional dyspnoea, and complained of difficulty in standing up after sitting, combing their hair, and buttoning their shirt.
Cutaneous examination this time revealed increased skin involvement compared to the initial presentation (Figures 1C and 1D), marked sclerodactyly on the right hand as compared to the left (Figure 2A), a beaked nose, a masked face, and a purse-string mouth (Figure 2B). The modified Rodnan skin score score was 32, and the European League Against Rheumatism (EULAR) score was 18.
Further investigations revealed: haemoglobin: 8 mg/dL; total leukocyte count: 9,830; platelet count: 455,000; serum glutamic pyruvic transferase: 11; creatinine: 0.7; urine routine microscopy: no abnormality detected; erythrocyte sedimentation rate: 47; C-reactive protein: 15.40; pulmonary function test: normal; and ECG: left bundle branch block. Dermoscopy revealed a white structureless area with erythema, accentuated pigment network, and loss of appendages (Figures 2C and 2D).
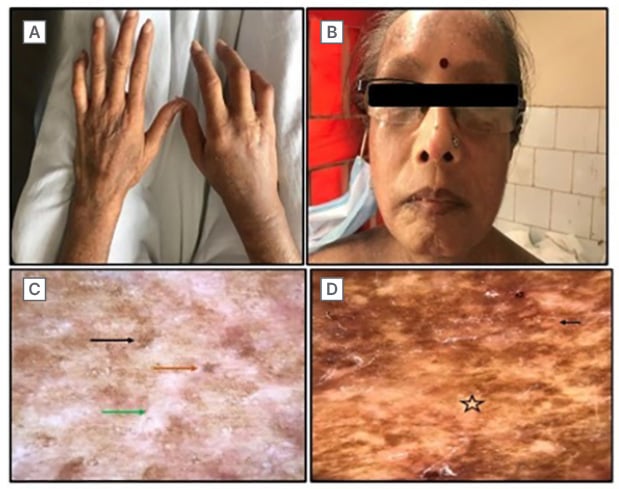
Figure 2: Facial involvement and dermoscopic examination from the site of biopsy.
A) Sclerodactyly markedly visible on the right hand with a scar of amputation. B) Shiny, wrinkle-free, mask-like facies with a beaked nose and purse string mouth. C) Polarised dermoscopy from the back. White structureless area (green arrow) with faint erythema loss of appendages (orange arrow), and accentuated pigmentary network (black arrow). D) Non-polarised dermoscopy. Faint erythema (black arrow), and white structureless area (star).
Histopathology showed thinning and effacement of rete ridges and basal layer hyperpigmentation, along with the presence of periappendageal and perifollicular lymphocytic infiltrates. Dermis displayed thickening and hyalinisation of collagen (Figure 3A and 3B). Nail fold capillaroscopy showed tortuous, regressing capillaries and avascular areas suggestive of SSc (Figures 3C and 3D). A past episode of chronic plaque psoriasis showed multiple well-defined erythematous plaques with scaling over extensors (Figure 4).
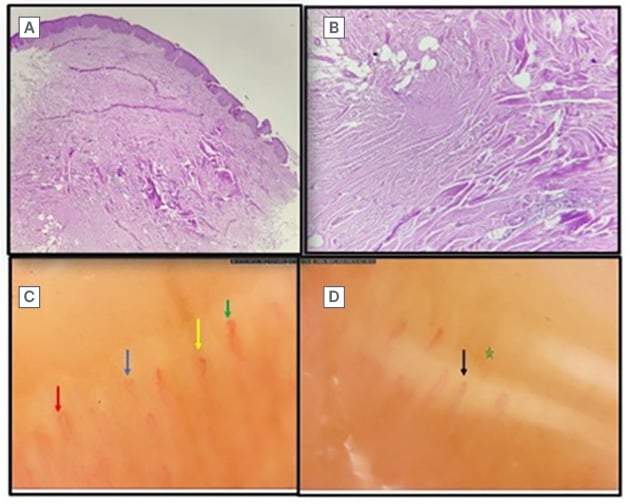
Figure 3: Histopathologic image and nail fold capillaroscopy.
A) Scanner view in 10x magnification showing thinning and effacement of rete ridges with basal layer
hyperpigmentation, perifollicular and periappendageal infiltrate consisting of lymphocytes and plasma cells. B) 40x magnification of the dermis showing thickening and hyalinisation of collagen with loss of epidermal appendages. C) Dilated capillaries (red arrow), bushy capillaries (blue arrow), meandering (green arrow), and micro-haemorrhage (yellow arrow). D) Receding capillaries (black arrow) with avascular zones (green star).
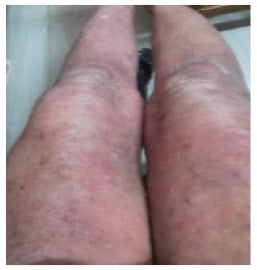
Figure 4: Well-defined erythematous plaques with scaling over extensors.
ANA profile was repeated and the U1 RNP antibody was mildly positive (1.14 units), with negative double-stranded DNA antibodies, anti-nucleosome antibodies, anti-histone antibodies, anti-Sjögren’s-syndrome-related antigen A autoantibodies, anti-Sjögren’s antibodies, Scl-70 antibodies, Mi-2 antibodies, anti-Ku antibodies, anti-centromere antibodies, and anti-PM-Scl antibodies.
The patient was referred to a rheumatologist for combined treatment, and was prescribed methotrexate 10 mg/week, mycophenolate mofetil 360 mg twice daily, and prednisolone 5 mg once daily. As the patient developed severe gastric intolerance to mycophenolate mofetil, they were very non-compliant on further visits.
As per the chronology, the patient was diagnosed with generalised morphoea post-radiotherapy during their first visit, as there were no systemic manifestations, and the lesion was not limited to the site of radiation. After a further 6 months, there was development of systemic symptoms, putting the authors’ diagnosis of generalised morphoea in dilemma; further, the diagnosis was kept as SSc with anti-U1 RNP positive antibody. The cause of SSc manifesting in the later age after radiotherapy was a matter of concern, whether it is a presentation of de novo SSc, or radiotherapy unmasked the antigens already present in the body.
DISCUSSION
Radiation-induced dermatologic manifestations are prevalent, with up to 90% of patients experiencing a local skin reaction. Within the first 2 months of exposure, the earliest changes are consistent with localised irritation and drying of the skin due to inflammatory cytokines. Fibrosis, telangiectasias, and skin necrosis are late effects, defined as 2 months–decades after the last radiation exposure.1 Post-irradiation morphoea would be classified as circumscribed morphoea with lesions confined to the radiated field and surrounding tissue, the majority described in breast cancer patients.
Bleasel et al.3 and Davis et al.4 each report a morphoea frequency of 1:500 per year in irradiated patients with breast cancer, and an incidence of 2.7:100,000 per year in non-irradiated individuals. The incidence differential strongly suggests that radiotherapy is a risk factor. However, only 81 instances of radiation-induced morphoea have been documented in scientific literature since 1989.5 In rare instances, the effects of radiation can extend beyond the irradiated area or even become generalised, defined as four or more indurated plaques on more than two of seven anatomic sites.5,6 Patients with generalised morphoea are more likely to have positive serology for autoantibodies, especially ANA.7
Histological evaluation is essential for differentiating diseases such as chronic radiation dermatitis, cancer recurrence, radiation recall dermatitis, newly-developed carcinoma, and cellulitis.8 In the burnout phase of early lesions, inflammation and infiltration predominate, whereas, in the sclerotising phase of late lesions, extensive fibrosis (thickened collagen fibres) predominates.8 Post-irradiation fibrosis only occurs within the irradiated field during the first 3 months of radiation, whereas radiation-induced morphoea can spread to other tissues.8
It has been proposed that radiation exposure may activate clonal fibroblasts in post-irradiation morphoea, leading to an increase in cytokine production, including that of transforming growth factor β.1 This cytokine has been connected to the pathophysiology of both localised and diffuse scleroderma.1 The increased cytokine response is thought to boost the production of glycosaminoglycans, collagen, and extracellular matrix proteins.1
Given the risk of radiation-induced fibrosis in the skin, lungs, and other organs, scleroderma is regarded as a relative contraindication for radiation therapy. As observed in this case, there are other case reports and case series suggesting that patients with scleroderma may develop exaggerated fibrosis, and it is commonly feared that radiation therapy could trigger a flare-up of systemic rheumatic disease.2
During the initial visit, the patient had plaque only limited over the trunk and absence of systemic complaints, so the diagnosis after the histological examination was kept as radiation-induced morphoea.
On the next visit, after 6 months, the patient had developed the systemic manifestation of SSc, along with typical facies and positive anti-U1 RNP antibody after almost 1.5 years of radiotherapy. Thus, the diagnosis kept by the authors was now questionable. The conundrum occurred between the diagnosis of de novo manifestation of SSc post-radiotherapy, an unmasking of underlying SSc secondary to radiation, and conversion of radiation-induced morphoea to SSc, as the patient had no symptoms or signs of localised or systemic scleroderma before the occurrence of SCC and subsequent radiotherapy.
Radiation-induced morphoea, conversion of localised scleroderma to systemic scleroderma,9 and de novo SSc post-radiation10 are infrequently described, severe, and unpredictable late side effects, with a wide range of onset times. As soon as histological confirmation is obtained, local and systemic therapy must be started to slow the progression of fibrosis and atrophy, and enhance the patient’s quality of life.
Based on the authors’ extensive evaluation and literature search, there are nominal reports in the present literature on the conversion of radiation-induced morphoea to SSc post-radiotherapy. Thus, by disclosing this unusual case, the authors want physicians to always raise suspicion and keep track of patient’s symptoms to avoid complications of the disease and manage it at the earliest.