Abstract
Background: The neonatal period is a phase of adaptation during which several skin conditions can develop. Most of these findings characterise the newborn’s skin, such as lanugo, erythema of the skin, and vernix caseous.
Objective: To describe the most common neonatal dermatological findings and classify them as transient neonatal skin conditions, congenital birthmarks, benign neonatal pustuloses, naevi lesions, and skin malformations.
Discussion: Skin changes are very common in neonates and span a vast range of conditions. This demonstrates the importance of good knowledge and awareness of newborn skin.
INTRODUCTION
The neonatal period comprises the first 4 weeks of life. It is a period of adaptation during which several skin conditions can often occur, from temporary lesions caused by physiological responses, to others resulting from transient diseases, and some as markers of severe pathologies.1-3 In their first days of life, newborn (NB) skin undergoes adaptation processes needed to accommodate the transition from the wet, aseptic, and homeostatic uterine environment to the dry atmosphere with temperature oscillation, surrounded by micro-organisms.4 Some authors have studied the prevalence of dermatological findings on NB skin and how it differs among distinct racial groups.5 It was found that >90% of NBs have some form of cutaneous manifestation and 84% have more than one single dermatological finding.6,7
NEWBORN SKIN CHARACTERISTICS
There are many important differences between adult, pre-term, post-term, and full-term skin (Table 1).8,9 The functional difference between adult skin and that of NBs is due to the microstructure of the skin layers. The stratum corneum is thinner, the cohesion and adhesion of epidermal cells weaker, there is less melanin production, the pH of the skin is higher, and the skin surface area/weight is higher in NBs.7,10,11 There is also major transepidermal water loss and a delay of the sudoral response which is believed to reflect the immaturity of the sympathetic nervous system. In the neonatal period, the most important function of the skin is thermoregulation and to act as a barrier against cutaneous infections.12 The neonatal cutis is more likely to develop certain skin diseases, such as irritant contact dermatitis.2,13
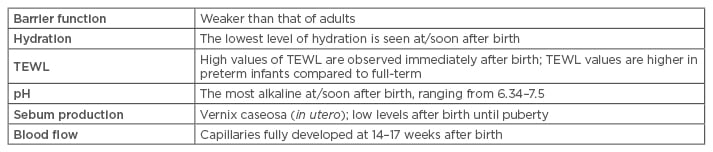
Table 1: Findings of newborn skin compared to that of adults.
TEWL: transepidermal water loss.
NEONATAL DERMATOLOGICAL FINDINGS
The main dermatological findings in NBs include lesions that are benign, such as transient physiological dermatological phenomena, those from the neonatal period (vernix caseosa, lanugo, etc.), phenomena due to maternal hormonal status (gynaecomastia, genital hyperpigmentation), dermatological manifestation of systemic conditions, developmental anomalies, and neonatal vesicopustular lesions. For better didactic organisation, we have classified these as follows: physiological dermatological phenomena; phenomena due to hormonal status; dermatological manifestations of systemic conditions; developmental anomalies; vascular anomalies; and neonatal vesicopustular lesions.
Physiological Dermatological Phenomena
These dermatological findings are found during the neonatal period itself and, most of the time, represent the neonate’s skin. The main dermatological findings within this group are described below:
Lanugo
Lanugo occurs in approximately 40% of NBs.14 It is a thin, soft layer of hair with little pigment and no medulla; it covers the NB skin and is more common and clearly seen in pre-terms. It is found on the dorsal trunk, shoulders, forehead, ears, and face of newborns. It should be considered in the differential diagnosis of congenital hypertrichosis lanuginose, a rare inherited disorder in which the lanugo is longer and darker.12
Vernix caseosa
This is an acidic lipid mantle produced by fetal sebaceous glands, composed of water (81%), lipids (19%), and protein (10%) naturally adhered to the NB skin.15,16 Hydration of the skin tends to be higher in infants who remain with vernix caseosa and the pH and erythema of the skin tends to be lower than in neonates with their vernix removed. Its distribution across the NB skin depends on the gestational age, type of delivery, sex of the NB, race, and exposure to meconium. The majority of premature NBs lack this protective biofilm. In a previous study, maintaining or withdrawing the vernix caseosa using a bath showed no difference in the axillary temperature of the neonate.17
Erythema
Erythema is seen in around 19% of neonates.14 It presents as bright red colouring all over the skin, usually seen within the first 24 hours of life. It occurs due to vasodilation of cutaneous capillaries, most likely caused by a decrease in sympathetic tone.18
Eyelid oedema
Observed in 17% of neonates within the first week of life, eyelid oedema occurs due to the increased pressure during birth, or due to the use of silver nitrate, and lasts 3–4 days.14
Milia
One of the most common transient skin conditions in neonates, milia presents in up to 30–50% of neonates. The milia consist of 1–2 mm yellow papules on the face, localised predominantly to the nose. It represents epidermal keratin cysts developing in connection with pilosebaceous follicles. When these cysts are seen on the palate, they are termed Epstein pearls and when on the alveolar margins, they are termed Bohn’s nodules.19 No treatment is required for neonatal milia as these spontaneously resolve within a few weeks. Extensive, persistent, and uncommon locations require attention, because this could be a manifestation of Marie Unna hereditary hypotrichosis, orofacial-digital syndrome Type I, Basan syndrome, or X-linked Bazex–Dupré–Christol disease.20
Desquamation
Desquamation is also a characteristic of NB skin and is one of the most common cutaneous findings in the neonatal period; it has been reported to occur in 12–65% of neonates.14 Along with sebaceous hyperplasia, this shows a significant correlation with maturity; desquamation is more prevalent in post-terms and sebaceous hyperplasia in full-terms.1
Suction blisters
Suction blisters are induced by vigorous oral suction of a portion of the body while the baby is still in the uterus. They are observed in 0.5% of NBs and are characterised by oval blisters or erosions which mainly affect the back of the hands, fingers, forearms, and lips.21 Other authors have reported that suction blisters account for approximately 4.5% of neonatal vesiculobullous injuries (Figure 1).22
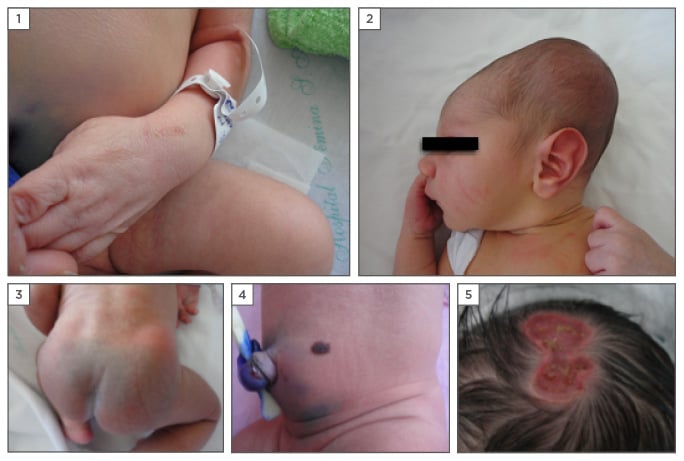
Figure 1: Paediatric dermatological findings presented at/shortly after birth.
1) Suction blisters on the hand; 2) caput succedaneaum; 3) Mongolian spot; 4) congenital naevus; 5) aplasia cutis congenita.
Caput succedaneum
This is a localised swelling on the scalp of infants that occurs due to the pressure of delivery. It is related to venous congestion and oedema resulting from the pressure of the cervix and is more prevalent in NBs of prolonged vaginal delivery and primigravida.7 Unlike with cephalhaematoma, caput succedaneum lesions often cross the midline and resolve spontaneously within 48 hours. Cephalhaematoma lesions do not cross the midline and are limited to a cranial bone (Figure 1).21
Mongolian spot or congenital dermal melanocytosis
A Mongolian spot or congenital dermal melanocytosis is a congenital birthmark with a collection of dermal melanocytes. It is clinically seen as a large macular lesion located over the lumbosacral area, buttocks, and occasionally the back, flanks, and shoulders of infants (Figure 1). It has been observed in 80–90% of black NBs, 91% of Asian children, 25% of NBs in Southern Brazil, and 20% of NBs within the USA.12,23-27 Congenital dermal melanocytosis usually disappears at around 4 years of age, but may persist; it depends mainly on any destruction that occurs within the protective extracellular fibrous sheath that covers the dermal melanocytes, and due to growth factors, the regulation in melanocyte proliferation, and genetic factors.5,28 Large and numerous congenital dermal melanocytosis lesions may be seen in lysosomal storage disorders, among them, Gangliosidosis Type 1 and Hunter and Hurler syndromes.29 Large and persistent Mongolian spots can be seen in phakomatosis pigmentovascularis in association with vascular malformations.30
Cutis marmorata
This is a physiological change evidenced by reticulated patches caused by dilation of capillaries and venules. It usually appears when the infant is exposed to low temperatures and disappears with heating, corresponding to an overreaction to hypothermia. It can last minutes to hours, both in premature infants and full-term NBs. Physiological marmorata cutis should be distinguishable from cutis marmorata telangiectatica congenita (which is a persistent vascular anomaly due to capillary malformation) and from reticular livedo (which can be observed in infants with neonatal lupus).12
Harlequin colour change
Harlequin colour change is benign, uncommon, and often observed in pre-term infants.27 It exhibits a sudden, brief change of skin colour, bordered on the midline, in which half of the body presents erythema and the other half pallor. Of unknown aetiology, this change in colour is believed to occur due to immaturity of the hypothalamic control of peripheral vascular tone.31
Miliaria
Miliaria rubra and crystalllina are both very common within the neonatal period and are caused by the obstruction of the exit of eccrine sweat from the gland ducts of the corneum stratum, with the subsequent retention of sweat. Miliaria rubra usually occurs in the second week of life and is characterised particularly by non-follicular erythematous papules, mainly in the intertriginous areas.32
Phenomena Due to Hormonal Status
Certain phenomena can occur mainly due to the passage of maternal and placental hormones. Most of the time, these are reversible.
Miniature puberty
Exhibiting changes that are similar during pregnancy and puberty, this may be observed as hyperpigmentation of the areola, the linea alba, and the external genitals. When this is accompanied by thickening and desquamation of the labia majora, with whitish or bloody discharge, it shows miniature puberty.12
Genital hyperpigmentation
Physiological melanic pigmentation fades away around the age of 2 years. It is seen in 19% of the NB population, and occurs at a higher rate in black skin.14
Neonatal acne
Facial inflammatory acne lesions, rash, and comedones can develop during the neonatal period. Hyperactivity of sebaceous glands and the stimulation of neonatal androgens are implicated in its pathogenesis.33 There is controversy over what truly represents neonatal acne, and pustular-papular conditions, characteristically without comedones, such as benign cephalic pustulosis (BCP) are characteristic of the neonatal period.34 Some authors consider BCP a neonatal acne variant caused by hypersensitivity to Malassezia furfur.30,35 Despite being a transient injury of the neonatal period, in some cases it may be present through the first months of life.21
Sebaceous hyperplasia
Sebaceous hyperplasia is a physiological manifestation of the NB period with a prevalence of 35–42.6%.24 It occurs in response to maternal androgen stimuli; this declines in the first trimester and only rises again during puberty.36
Dermatological Manifestations of Systemic Conditions
Jaundice
This occurs due to the immature liver’s inability to process excess bilirubin. In the skin, it reveals as a yellowish hue. Neonatal jaundice can be observed in ≤60% of term and 80% of premature NBs; the peak of incidence is after the third day of life and its intensity decreases progressively. It is important to differentiate this condition from non-physiological causes of jaundice.12
Infections
Many skin disorders presenting at or soon after birth can mimic infectious diseases. Pustules on NB skin can indicate a congenital or non-congenital infection. The infection cannot be diagnosed on the cutaneous symptom itself; it depends on other signs such as muscular tone, hepatosplenomegaly, organ-specific deficit, and the general appearance of the NB.37,38
Developmental Anomalies
Some anomalies in development can occur in the skin and this is observed throughout the neonatal period. Usually, these involve the head, nose, pre-auricular region, cervix, or spine, some in more than one location. Those that occur in the midline, especially involving the head, the nose, and the spine, can be more severe because of possible connections to the central nervous system.39
Congenital melanocytic naevi
Congenital melanocytic naevi are a specific type of pigmentary lesion that consists of the proliferation of nested melanocytic cells or naevi cells of neural origin. Approximately 1% of NBs have a congenital melanocytic naevus;31 American authors have reported an incidence of 2.4%.24 The risk of evolution to neurocutaneous melanosis or melanoma depends on its location and size (Figure 1).40
Naevus sebaceous
Naevus sebaceous consists of an epidermal naevus whose main component is sebaceous glands. The sebaceous naevus is an uncommon birthmark, seen in 0.3% of NBs.25 It is usually a single lesion, well defined, round, oval, or linear with a yellowish-pink colour, located on the scalp, face, or neck leather. There are three roots of these naevi: sebaceous glands stimulated by maternal androgen (at birth), those without a local stimulus, and sebaceous glands stimulated during puberty and gland enlargement.
Naevus achromicus or naevus depigmentosus
This presents as a hypopigmented spot, usually small and oval, but can also be extensive and follow the lines of Blaschko on the skin. These persist indefinitely and are more visible in the summer months and in patients with darker skin pigmentation.12 A differential diagnosis must include conditions such as naevus anemicus, ash-leaf spots, and vitiligo.41
Cephalocoele
This condition involves the protrusion of intracranial structures through a defect in the skull and is due to abnormal separation of the neuroectoderma from the ectoderm in the beginning of pregnancy. Cephalocoele is clinically characterised by a bluish compressible mass, usually located in the occipital region.39
Dermoid cysts
Dermoid cysts are characterised by smooth nodules that are non-compressible, skin coloured, and usually located in the occipital region, nasal dorsum, and spinal cord. It can cause infection and/or meningitis if there is communication with the central nervous system.39
Aplasia cutis congenita
Aplasia cutis congentia presents as an absence or thinning of the epidermis, dermis, and subcutaneous tissue; it affects 3 in every 1,000 NBs.39 It can be present as single or multiple lesions, alone or associated with other malformations, and most often affects the vertex of the scalp. According to clinical presentation, it can be divided into: aplasia cutis membranous, aplasia cutis irregular or stellate (usually non-membranous), aplasia cutis associated with embryonic alterations of internal organs, and aplasia with congenital absence of the skin (Figure 1).12
Amniotic constriction band
These are constricting rings that can affect the fingers, the extremities, and rarely, the cervical and trunk area; it is uncommon, occurring in 1 in 10,000 NBs.39 It is generally sporadic, although familial cases have been reported. It is believed that this anomaly is the result of an early rupture of the amnion where the amniotic liquid is leaked, leading to introduction of the fetus within the chorionic cavity. The corium and the reabsorbed fluid stimulate the proliferation of mesodermal bands that compress the fetal structures.28
Accessory tragi
This is a small papule, skin coloured, and located in the pre-auricular region which may be unilateral or bilateral. In most cases, it is not associated with any other anomalies.39
Adnexal polyp
Adnexal polyps are congenital and benign neoformations, usually solitary, about 1 mm in diameter, skin-coloured or slightly darker; they are located around the nipples. These resolve spontaneously, usually within 4 weeks of birth.42
Ichthyosis
A rare genetic disorder of epidermal cornification, this is rarely associated with any other syndrome, but could be a manifestation of Netherton syndrome or Refsum disease. Clinically seen as epidermal thickening, scaling, and cutaneous inflammation,43,44 it can be preceded at birth as a collodion baby, referring to a membrane covering the NB skin with variable degrees of ichthyosiform erythroderma, with or without superficial blisters.45 At birth the most common types are X-linked recessive ichthyosis, lamellar ichthyosis, and bullous congenital ichthyosiform erythroderma. The harlequin ichthyosis is the most rare and severe type of lamellar ichthyosis, with high mortality rates.46
Vascular Anomalies
Vascular anomalies can be classified into vascular tumours (benign, aggressive/borderline, and malignant) and vascular malformations including simple, combined, or major vessels, and those associated with other anomalies.47
Salmon patch or naevus simplex
This is the most common small cutaneous capillary malformation.47 The salmon patch occurs in 70% of white NB skin and 59% of black NB skin, most often located on the glabella (angel kiss) and neck (stork bite).5 A recent prospective study reported the presence of salmon patch in 83% of NBs within 48 hours of life.21 It fades in the first 2 years, however it can persist in rare cases.48
Naevus flammeus or port wine stain
Naevus flammeus is also a cutaneous/mucosa capillary malformation and can be associated with an underlying syndrome such as Sturge–Weber. It occurs in about 3 in 1,000 individuals.49
Neonatal Vesicopustular Lesions
Neonatal vesicopustular lesions are described as non-infectious or sterile pustules presenting within the neonatal period. They are benign cutaneous findings, self-limited, asymptomatic, and only occur during this period of time.37
Erythema toxicum neonatorum
Erythema toxicum neonatorum (ETN) develops as small erythematous papules, sterile vesicles, and pustules affecting the trunk, the extremities, and the face. Lesions usually appear on the second day of life and regress in 5–14 days.50,51 ETN can affect 30–70% of NBs, most of them born at term.52 The frequency of ETN may increase with increasing gestational age.53 Studies show that in full-term neonates its prevalence ranges from 30–50%, yet is only seen in 5% of pre-terms, with no statistically significant change in different races or sexes.28 It has recently been highlighted that there is activation of immune cells within the ETN lesions, suggesting an inflammatory reaction to the skin’s microbial colonisation at birth.51 The Tzanck test, conducted on a pustule, revealed the presence of eosinophils. In a prospective study, ETN was observed in 25.3% of NBs and was more prevalent in males.54 In 84.2% of allergic manifestations during the first 2 years of life, previous ETN or low pH was observed at birth; atopic dermatitis occurred in 85.7% of patients with previous ETN.54
Transient neonatal pustular melanosis
TNPM is presented at birth by flaccid and superficial pustules that break easily, forming a collaret scale and evolving into hyperpigmented macules of residual character. It occurs in 5% of black-skinned NBs and in <1% of white-skinned NBs.32 All areas of the body can be affected, including the palm and soles. Hyperpigmented macules with vesicopustules are characteristic of TNPM.55 Examination of the pustules using the Tzanck test showed the presence of polymorphonuclear neutrophils.54
Benign cephalic pustulosis
The cause of this neonatal skin colonisation by Malassezia spp. and the appearance of neonatal cephalic pustulosis in neonatal acne eruptions is uncertain. Authors have shown that colonisation increases significantly with the age of the neonate (5% in the first week, 30% from the second to the fourth weeks of life). Recent publications show that, although the skin colonisation increases after the first week of life, there was no correlation between cephalic pustulosis neonatal and Malassezia spp.56
CONCLUSION
The aim of this article was to provide a review of the main neonatal dermatological findings. NB skin is unique not only for its structure, but also its dynamics with the surrounding environment. Some of the knowledge about structural and physiological NB skin has changed over the years; we emphasise that the weaker barrier function is due to the immature cells and its cohesions. We reported some of the most common NB skin findings within the literature thus far by classifying them in a more didactical way. Earlier studies reported different routes of pathogenesis from the ones we now see, such as with BCP and the Malassezia spp. With regard to this scenario, we have seen the evolution of neonatal dermatology, and we look forward with a hope to contribute new findings within this vast area.







