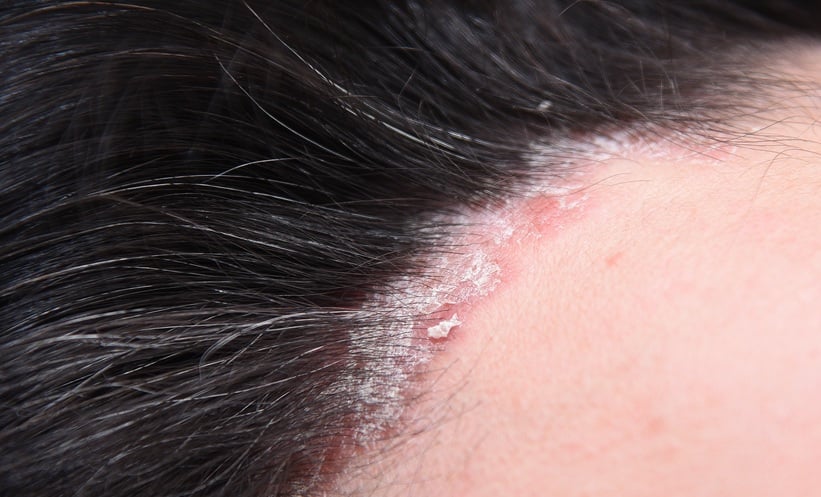
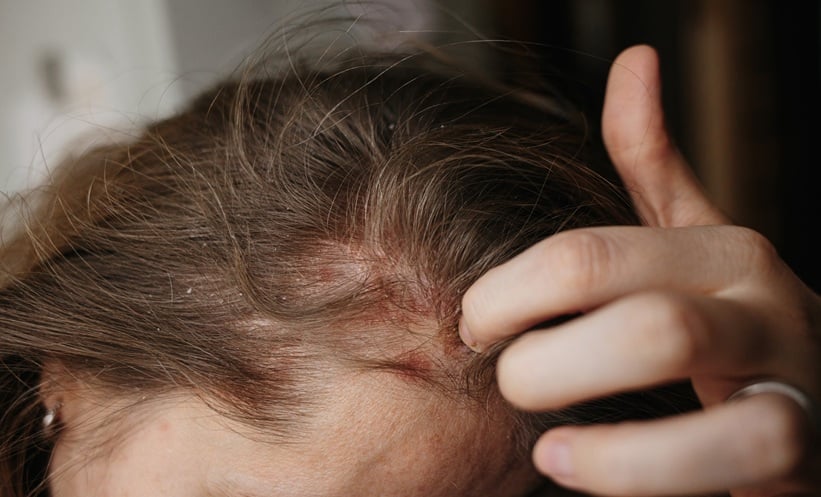
SCALP psoriasis, a challenging and often distressing condition, affects up to 80% of individuals with plaque psoriasis and is associated with symptoms such as pain, itching, flaking, and, in some cases, hair loss and pigmentation changes.
These effects can extend beyond the scalp, affecting visible areas like the neck and face, and can cause significant social, emotional, and functional impairments. The burden is especially high for individuals with skin of colour, including Black and Asian populations, where hair care practices and textured hair can complicate treatment. To address this gap, the VISIBLE trial was designed to evaluate guselkumab in patients with moderate to severe scalp psoriasis, specifically in individuals with skin of colour.
VISIBLE Cohort B assessed the safety and effectiveness of guselkumab in a diverse population with moderate to severe scalp psoriasis. By week 16, guselkumab was significantly more effective than placebo across all key clinical endpoints. By week 48, the average improvements in scalp-specific severity assessments exceeded 94%, with nearly 70% of participants achieving complete clearance of scalp psoriasis. Participants also experienced sustained and meaningful improvements in itch, overall symptom severity, and quality of life.
These findings build on the pivotal VOYAGE 1 and 2 trials, with VISIBLE participants generally presenting more severe baseline scalp disease and lower prior exposure to systemic treatments. Despite these challenges, guselkumab showed robust efficacy, even among participants who had been undertreated, likely due to healthcare disparities or hesitation to use biologics for scalp-dominant disease.
Itch, identified as the most severe and burdensome symptom, improved markedly with treatment. After just three doses, itch scores decreased to mild levels, and nearly half of the participants reported no impact of the disease on their lives. The safety profile remained consistent with previous studies, with common adverse events including respiratory infections and COVID-19.
VISIBLE Cohort B represents a landmark study in psoriasis research, being the only scalp psoriasis trial exclusively including individuals with skin of colour. Its inclusive design and real-world relevance offer valuable insights and reinforce the importance of tailored treatments for diverse patient populations.
Reference
McMichael A et al. Guselkumab for moderate to severe scalp psoriasis across all skin tones: cohort B of the VISIBLE randomized clinical trial. JAMA Dermatol. 2025;DOI:10.1001/jamadermatol.2025.1849.