ACNE has emerged as a frequent and clinically relevant side effect in patients with inflammatory bowel disease (IBD) treated with JAK inhibitors, according to the largest international retrospective cohort study to date on this adverse event.
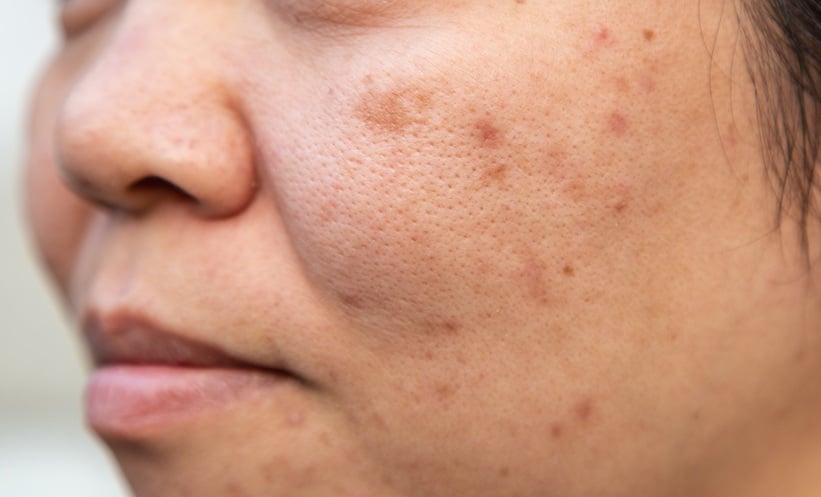
In a multicenter analysis involving 2,183 patients with IBD receiving JAK inhibitors, researchers found that 272 patients, approximately 12%, developed acne, with notable variation across agents. Upadacitinib was associated with the highest crude prevalence of acne (15.9%), followed by tofacitinib (4.3%) and filgotinib (1.9%). A dose-response relationship was identified for upadacitinib and tofacitinib, underscoring the potential for exacerbated dermatologic effects with higher exposure.
Patients aged 30 to 50 years were most frequently affected, with the majority presenting with mild to moderate acne. However, a prior history of acne vulgaris significantly increased the likelihood of developing severe acne and associated skin complications. Specifically, these individuals had nearly five times the odds of severe presentations (OR 4.88, 95% CI 2.88–31.7; p=0.0003) and nearly four times the odds of experiencing acne-related dermatologic complications (OR 3.92, 95% CI 1.56–10.11; p=0.004).
Psychosocial impact was also reported, with one-third of patients experiencing negative effects. Forty percent received pharmacologic treatment for their acne, and 18% required JAK inhibitor dose adjustment or discontinuation, though most of these patients did not exhibit severe acne.
This study is the first to comprehensively describe the clinical characteristics and treatment outcomes of JAK inhibitor-induced acne in IBD. The findings support the need for proactive dermatologic assessment and patient counseling before and during JAK inhibitor therapy. Early recognition and appropriate intervention may improve adherence and minimize disruption to IBD management.
Reference:
Honap S et al. Janus kinase (JAK) inhibitor-induced acne in inflammatory bowel disease – an international, multicenter, retrospective cohort study. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00465-3.