Meeting Summary
Dermatologists today have more tools than ever at their disposal for managing psoriasis, including newer-generation biologics, which target molecular drivers of psoriatic inflammation that were scarcely known a decade ago. With a deeper understanding of epidermal immunology, we now recognise key pathogenic roles for multiple cytokines and cytokine receptors. Among current treatment targets, we now include not only the tumour necrosis factor pathway, but also interleukins (IL) of the IL-17 family (which are produced by T helper 17 [Th17] cells, among other skin cells) and IL-23 (which polarises the immune response toward Th17 production), as well as the corresponding receptors and intracellular signalling molecules. Rapid and complete skin clearance has become increasingly feasible, as suggested by studies of the newer biologics, including ustekinumab (targeting Th1 and Th17 cell development), secukinumab and ixekizumab (targeting the IL-17A cytokine), and brodalumab (targeting the IL-17 receptor subunit A). Paralleling the improved skin-related outcomes, we see a lightening of the burden of disease that patients experience with this chronic condition.
The bar is being raised when it comes to treatment goals investigated as endpoints in Phase III trials, and we see a shift from control to partial, or even complete, clearance. A similar evolution toward ambitious and personally tailored treatment goals is needed in the clinic. The speakers in this symposium addressed the promise of the new approaches, and the continuing challenge of choosing the optimal therapeutic approach, to ensure that each patient gets the best results from their therapies, whether old or new.
New Treatments, New Data: Rethinking Future Treatment Goals
Professor Kristian Reich
Rethinking the Model of Psoriasis Pathogenesis
“Take a fresh look at the IL-17 world,” suggested Prof Reich, kicking off this symposium on the future of psoriasis treatment. As an immunologic skin disease, the pathogenesis of psoriasis is traditionally explained by cytokine-mediated crosstalk between dendritic cells and T cells residing in the dermis and epidermis.1-4 Much clinical research has focussed on cytokines of the IL-17 family and on Th17 cells, which produce IL-17 and appear to collaborate with Th1 cells to drive the characteristic epidermal changes seen in psoriasis. Recently, it has emerged that Th17 cells are less common in active psoriatic plaques than would be expected in this model,1,2,4,5 suggesting that other IL-17-producing cell types might contribute more to the psoriasis disease process than previously thought.1,4,6 Indeed, skin keratinocytes release two members of the IL-17 family cytokines, IL-17C and IL-17E, which can trigger an immune response alongside IL-17A.7-11
Whatever their cellular source, the IL-17 proinflammatory cytokines transduce their signals through receptors sharing the IL-17 receptor subunit A (IL-17RA), the molecular target of the biologic agent brodalumab.6 Because brodalumab binds to IL-17RA, this human monoclonal antibody inhibits signalling not only by IL-17A, but also by IL-17F, IL-A/F heterodimer, IL-17E, and IL-17C.6
The Brodalumab Clinical Development Programme
Brodalumab has been studied in one of the largest clinical trial programmes for a new biologic for psoriasis, with 4,464 patients treated in 17 Phase I–III clinical trials.12-21 From the 8,655 patient-years of brodalumab exposure and 9,174 patient-years of follow-up so far reported,19-21 most of the available data come from the AMAGINE programme, consisting of three Phase III studies.22,23
AMAGINE-1 was a double-blind, placebo-controlled study that tested the efficacy and safety of brodalumab on 661 adults with moderate-to-severe psoriasis.24 Patients were randomised to brodalumab (140 mg and 210 mg) or placebo every 2 weeks, with an additional dose at Week 1, during the initial 12-week induction phase. During this symposium, the focus was on the results obtained with 210 mg administered every 2 weeks, as this is the approved label dosage.
At Week 12, patients meeting the co-primary endpoint of static Physician’s Global Assessment of 0 or 1 (sPGA success) were re-randomised to either continue on their current dose or to transition to placebo treatment.24
AMAGINE-2 and AMAGINE-324 were two identically designed head-to-head Phase III studies in which patients were randomised to receive brodalumab (140 mg and 210 mg), ustekinumab (as per label),14 or placebo. At Week 12, patients receiving brodalumab in the induction phase were re-randomised to receive a brodalumab maintenance dose of 210 mg every 2 weeks (label dose) or various other maintenance doses.24 Patients receiving ustekinumab continued to receive ustekinumab every 12 weeks, and patients receiving placebo were transitioned to 210 mg of brodalumab every 2 weeks.
Clinical Efficacy and Patient Health-Related Quality of Life
In AMAGINE-1, 83.3% of patients treated with 210 mg of brodalumab achieved Psoriasis Area Severity Index (PASI) 75 by Week 12, and 75.7% achieved the co-primary endpoint of sPGA success.22 However, it could potentially be argued that PASI 75, which implies that patients may continue to experience substantial residual disease, no longer suffices as a treatment goal. In this regard, the more stringent secondary efficacy endpoints of PASI 90 and 100 are of interest.
In AMAGINE-1, 70.3% of patients treated with 210 mg of brodalumab achieved PASI 90, and 41.9% achieved PASI 100 (or completely clear skin) at Week 12.22 At Week 52, 83.1% of patients who were re-randomised to receive 210 mg brodalumab maintained sPGA success.
This trial setting partially simulated daily clinical practice, in that AMAGINE-1 patients were not allowed to experience a total relapse of the disease. Rather, they were retreated following loss of response, defined as sPGA 2 for at least 4 weeks or sPGA ≥3 at one visit.22 With retreatment, 97% of patients receiving 210 mg brodalumab recaptured sPGA success (0 or 1) after 12 weeks of retreatment.22 The PASI 100 response at Week 52 was 67.5% throughout the study in patients on 210 mg brodalumab and who achieved sPGA success (0 or 1) at Week 12 (Figure 1). These results should be seen in context of the study’s conservative imputation methodology, whereby patients missing data were assumed to be non-responders.
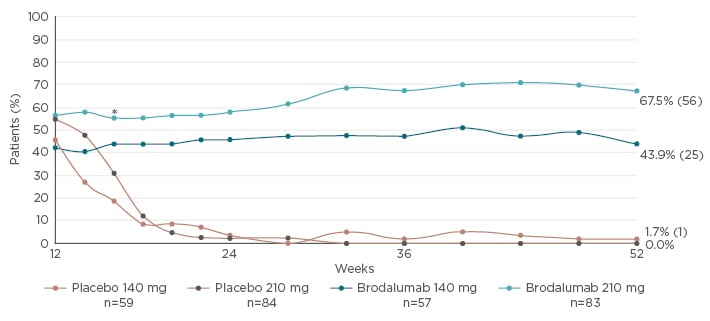
Figure 1: PASI 100 response rates through Week 52 in patients who achieved sPGA success (0 or 1) at Week 12 (AMAGINE-1).
All p-values <0.001 for comparisons between brodalumab and withdrawal (placebo) groups at all time points from Week 16–52; *p=0.015 between brodalumab 140 mg every 2 weeks and placebo. Treatment groups were as planned for induction/withdrawal phases, using modified intention-to-treat analysis. Non-responder imputation was used for missing PASI data.
PASI: Psoriasis Area and Severity Index; sPGA: static Physician’s Global Assessment.
Adapted from Papp et al.22
In AMAGINE-2 and AMAGINE-3, significantly more patients achieved PASI 90 at Week 12 with brodalumab 210 mg (70% and 69%, respectively) than with ustekinumab (47% and 48%, respectively).23 PASI 100 response was also significantly higher with brodalumab 210 mg (44% and 37%, respectively) than with ustekinumab (22% and 19%, respectively).
By Week 12, >55% of AMAGINE patients treated with 210 mg of brodalumab achieved Dermatology Life Quality Index (DLQI) 0 or 1 (55.9%, 60.8%, and 59.0% in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively), meaning that the patients were no longer burdened by the disease.25 Patients were also followed using the Psoriasis Symptom Inventory (PSI), which queries patients on their experience of itch, redness, scaling, burning, stinging, cracking, flaking, and pain.22,23,26 Researchers calculated the proportion of symptom-free days, defined as the ratio of days the patients reported a PSI total score of 0 and the total number of days with PSI assessments available.27
As expected, there was a clear relationship between these patient-reported outcomes and the clinical findings. Thus, 80% of responders who achieved PASI 100, but only 63% of patients with a PASI response between 90 and 100, reported a DLQI of 0 or 1.27 Likewise, 42% of responders who achieved PASI 100 reported being symptom-free each day between their final visit, Week 12 visit, and the previous visit, compared to 14% of responders whose PASI response was between 90 and 100.
Safety Profile of Brodalumab
When considering patient treatment with biologic therapies that target the IL-17 pathway, a number of safety events, including Candida infections, worsening of Crohn’s disease in subjects with active Crohn’s disease, and neutropenia are highlighted by experts as identified risks. Safety results from AMAGINE-2 and AMAGINE-3 indicate that these specific safety concerns and other potential safety risks of interest occurred at rates that were consistent with those observed with other biologics and were comparable between ustekinumab and brodalumab.23
Suicidal ideation and behaviour (SIB) represents a potential risk for the target population, since rates of depression, anxiety, and suicidal ideation are significantly elevated among individuals with psoriasis.28 In the AMAGINE programme, 52-week follow-up, time-adjusted patient incidence rates for SIB were 0.20 and 0.60 per 100 patient-years for brodalumab and ustekunimab, respectively.25 In the long-term pool of patients treated with brodalumab, there were 4 cases of completed suicide, 1 of which was later adjudicated as indeterminate.29 These 3 patients showed no consistent pattern between exposure and the timing of suicidal behaviour, and all three cases featured recent and/or longstanding depression or other significant life-stressors.25 Notably, in the background population, the probability of four suicides occurring in a group the size of the AMAGINE programme over the same time-frame is 26%, and the probability of three suicides rises to 47%30 (assuming a rate of 0.028 per 100 subject-years based on a meta-analysis of published clinical trials and/or registries for psoriasis).31
There has been no mechanistic explanation established between IL-17 pathway inhibition and SIB.32 Because patients with a history of drug abuse, depression, suicidality, or other psychiatric comorbidities were not specifically excluded from the AMAGINE programme,33-35 it may be hypothesised that this trial population presents a higher risk for psychiatric comorbidities than in some comparable studies.
Upon review of these data, neither the European Medicines Agency (EMA)36 nor the U.S. Food and Drug Administration (FDA)30 identified a causal link between brodalumab treatment and an increased risk of SIB. Indeed, patients treated with brodalumab showed a significant reduction in hospital anxiety and depression scale scores at Week 12 (p<0.001 for comparisons between brodalumab and placebo groups).22 Brodalumab currently has market approval in the European Union (EU), USA, and Japan.
Conclusions
Overall, the evidence we base our treatment decisions on increasingly suggests that the treatment goal of a life unburdened by psoriasis is both feasible and desirable. Targeting the IL-17 pathway with brodalumab yielded high initial levels of disease clearance that were maintained over ≥52 weeks of treatment. Individuals who achieved clearance or complete clearance were markedly more successful than others in eliminating the disease burden associated with moderate-to- severe psoriasis.
Beyond the Skin: A Broader View on Disease Facets
Professor Caitriona Ryan
Using two hypothetical patient cases, Prof Ryan highlighted different facets of the disease and the impact of comorbidities. Data from the brodalumab development programme were used to illustrate the potential benefits of targeting the IL-17 pathway in the patient-centred management of psoriasis. Both cases featured treatment-experienced patients with moderate-to-severe psoriasis, whose disease was insufficiently controlled despite ongoing treatment with a biologic.
The first case highlights the need for rapid control in a patient expressing desire for complete clearance. The second illustrates the possibility of effective clearance of psoriasis in a middle-aged patient with personal and familial risk factors for cardiovascular disease.
Case 1: A Patient Requiring Rapid Onset of Action
The first case described a 21-year-old female university student who was experiencing a psoriatic flare that commenced 18 months after initiating adalimumab, with active plaques on her knees, elbows, stomach, and back, as well as inframammary involvement. Body-surface area involved was estimated at 10%. Her poor disease control was a source of anxiety, particularly because she was to be a bridesmaid for her sister’s wedding 1 month later.
There was no joint pain and the patient was otherwise healthy. Previous treatments included methotrexate, which she did not tolerate, and phototherapy, which produced a very short duration of remission. She was taking adalimumab at the recommended dose.
Options for achieving control quickly in this patient may have included addition of a topical agent to clear obvious areas, combination treatment with a systemic treatment, or switching to another biologic therapy. In this case, an anti-IL-17 treatment was initiated. At Week 2, the patient achieved <1% body-surface area, with near clearance of psoriasis and only small residual plaques on her back.
Rapid onset of action
This outcome, including the rapid achievement of clinically significant disease control, is in line with findings in recent clinical studies of brodalumab in patients with moderate-to-severe psoriasis. At Week 1, a significantly higher number of patients achieved PASI 75 with brodalumab than with ustekinumab.37 When treated with brodalumab, the median time for 25% of the patients to achieve a PASI 75 response was 2.14 weeks, whereas with ustekinumab, the median time required for the same response was 4.77 weeks.37 Moreover, the median time to PASI 90 response was shorter in the brodalumab group (6.43 weeks) compared with ustekinumab (12.1 weeks).38 PASI 100 response was also shorter in the brodalumab group (12.4 weeks) compared with ustekinumab, where the minimum for PASI 100 was not reached within 12 weeks.38 More than half of patients treated with 210 mg brodalumab throughout the 52 weeks achieved PASI 100 (56% and 53%, respectively, in the AMAGINE-2 and AMAGINE-3 studies) at 52 weeks.23
The follow-up plan
For this patient, the clinical decision was to continue treatment as long as she maintained clearance or near clearance of her disease. “With a new generation of biologics, the bar has been raised,” Prof Ryan said. “Our patients deserve to be cleared of their psoriasis.”
Case 2: A Patient with Cardiovascular Risk Factors and a History of Treatment Failures
A 56-year-old salesman had suffered from psoriasis for many years, affecting his face, hands, and genital area, as well as nail involvement. He was a one-pack-a-day smoker with a history of hypertension and a family history of cardiovascular disease. Previous treatment with adalimumab had failed, and he had used ustekinumab for the previous 2 years. However, this patient had never achieved clearance of his facial or genital psoriasis.
Visible disease had lowered his confidence in front of customers, a situation that was affecting his performance at work. There was genital involvement including lichenified, itchy plaques on the shaft of the penis and the entire scrotum. The presence of genital disease was greatly affecting his sexual life with his partner. Because of the potential for profound adverse effects on health-related quality of life and sexual health, patients with genital psoriasis should be specifically asked about their burden of disease, as they may be reticent to volunteer this information.39
Considering comorbidities
More than any other dermatologic condition, psoriasis has been associated with depression, anxiety, and SIB risk.28,40 Severe psoriasis is also associated with a 50% increased risk of mortality compared with the general population, with cardiovascular death as the most common aetiology.41,42 When choosing a treatment for a patient, Prof Ryan advised to carefully consider the myriad of conditions associated with psoriasis, including cardiovascular and affective comorbidities.43 For this case, the treatment plan needed to aim at minimising risk of major adverse cardiac events, as he was a smoker, had high blood pressure, and had a family history of cardiovascular disease.
The treatment options for this patient included continuing his current therapy, possibly with an increased injection frequency (off label treatment), or a switch in biologic therapy. The decision was made to initiate an anti-IL-17 treatment.
Prior treatment history
Although previous failure on biologic treatment might suggest a poor prognosis with subsequent therapies, brodalumab has shown similar efficacy in biologic-naïve patients as it has in biologicexperienced patients.37 Treatment with brodalumab allowed 32% of the patients who experienced unsuccessful previous biologic therapy to achieve PASI 100 at Week 12, compared with only 11% with ustekinumab (p=0.0027). In addition, patients with inadequate response to ustekinumab after 16 weeks rapidly achieved and maintained high levels of skin clearance for ≤36 weeks after receiving brodalumab 210 mg rescue therapy; 38% of patients achieved and maintained PASI 100.44
A quantitative summary of clinical findings at 52 weeks (Figure 2) demonstrated superior efficacy of brodalumab, relative to ustekinumab, with a similar safety profile.
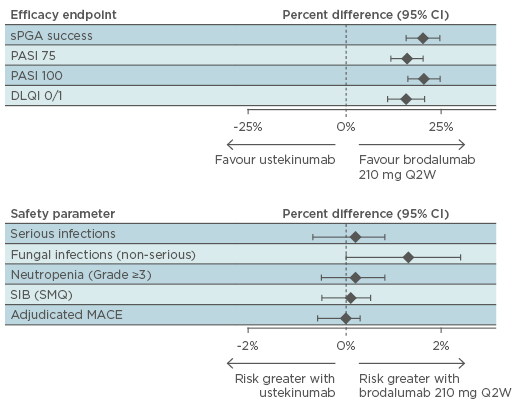
Figure 2: AMAGINE-2 and AMAGINE-3: Benefit-risk summary plot at Week 52 (ratio comparison).31
CI: confidence interval; DLQI: Dermatology Life Quality Index; MACE: major adverse cardiac events; PASI: Psoriasis Area and Severity Index; SIB: suicidal ideation and behaviour; SMQ: Standardised MedDRA. Query; sPGA: static Physician’s Global Assessment; Q2W: every 2 weeks.
The follow-up plan
Having initiated brodalumab in this patient, it is essential to ensure that his facial and genital psoriasis are controlled. His cardiovascular risk remains a concern. Smoking cessation is a priority, as is ongoing monitoring and treatment of hypertension.
Which Drug for Which Patient? The Question of Patient Profiling
Professor Hervé Bachelez
With today’s array of newly-developed biologic treatments, clinicians have a wide choice of therapies for their psoriasis patients. Ambitious treatment goals, which have become increasingly feasible with newer treatments, may be key to providing patient outcomes in line with patient expectations. However, to involve patients appropriately in the decision-making process,45 dermatologists must explain the benefits and risks of the various options clearly; even today, the dermatologist remains a patients’ primary source of such information.
The scarcity of real-life data on biologics complicates treatment decisions; therefore, psoriasis patient registries are a potentially valuable resource. In the absence of extensive real-world evidence, European guidelines for treatment of psoriasis are driven by consensus and expert opinion to establish recommendation for clinicians.46,47 Registries can fill a gap by providing evidence about patients whom we encounter in daily practice but who might be excluded from clinical trials.
Achieving Skin Clearance Safely: A Fine Balance
With the anti-IL-17 agents, an increasing number of patients can reach complete or almostcomplete clearance, making PASI 90 and even PASI 100 an achievable therapeutic goal.47-49 The odds of a patient reaching PASI 90 or PASI 100 are highest with anti-IL-17 biological agents, according to a network meta-analysis presented at this year’s European Academy of Dermatology and Venerology (EADV) congress, which modelled the probability of PASI responses for biologics approved in the EU.50 Patients have the highest likelihood of achieving PASI 100 when treated with brodalumab, ixekizumab, or secukinumab (Figure 3).
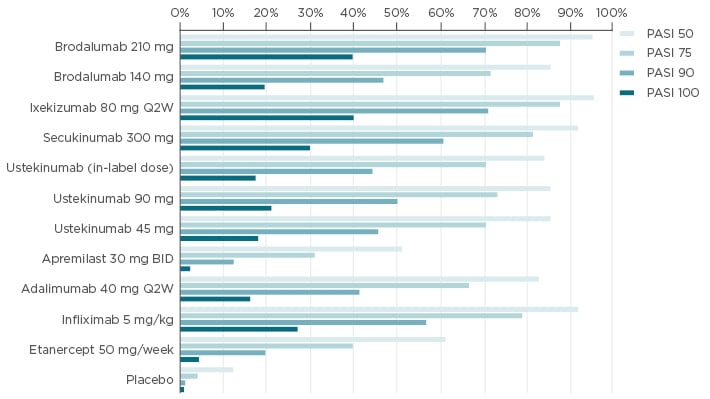
Figure 3: Meta-analyses of expected probabilities of achieving different treatment outcomes.50
Probabilities estimated based on the unadjusted network meta-analysis.
BID: twice-daily; PASI: Psoriasis Area and Severity Index; Q2W: every 2 weeks.
When considering data supportive of treatment safety, registry data will be an invaluable, but not a sovereign, source of data. Important safety issues remain unanswered in randomised clinical trials, and there is a need for newer methods to assess rare risks.51,52 Moreover, the limited patient population size of clinical trials may lead to failure to discriminate rare adverse drug reactions from background incidence rates. Registry studies could provide such insight, thanks to the larger samples of patients available.53 Even so, registry findings will require cautious interpretation, as safety signals do not themselves establish a causal link with a given treatment.
Patient priorities will, therefore, remain a crucial factor in deciding among therapies. These priorities may differ somewhat from those of dermatologists. The Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey shed light on physicians’ concerns about biologics’ long-term safety when initiating or pursuing treatment.54 Unlike physicians, patients in the MAPP survey expressed little fear of adverse events, but were concerned about tolerability and safety issues that might lead them to discontinue treatment.55
Inflammatory bowel disease (IBD) provides an interesting example of the difficulty of evaluating safety in the face of rare events. Isolated cases of new-onset IBD have been observed in the development programmes of all three IL-17-blocking biologics. One confirmed case of IBD (Crohn’s disease) occurred in the brodalumab psoriasis development programme,24 and 19 cases of IBD, mostly de novo, were adjudicated (12 of ulcerative colitis and 7 of Crohn’s disease) among 4,209 patients exposed to ixekizumab.13,56 Three cases of IBD (anal fistula, ulcerative colitis, and Crohn’s disease) were identified in the secukinumab development programme.11,57 Interestingly, only brodalumab carries a contraindication for use in patients with active Crohn’s disease.24 Considering that patients with severe psoriasis are at elevated risk of IBD,58 the occurrence of IBD cases in these clinical trials inevitably raises the question of causality. Data collected so far provides no clear answers, and careful interpretation of results should be observed.
First-Line Therapies
The selection of a first-line biologic treatment for psoriasis remains a moving target, with no single choice right for all patients. Physicians appear to make this decision on the basis of evidence and expert opinion, taking into account patient factors such as age, cardiovascular risk, tuberculosis screening results, and comorbidities, including joint involvement.59 There is an undeniable need for real-life registries, which, with robust statistical methodologies, can provide the missing data necessary to inform patient and clinician decisions regarding treatment options.
Conclusions
Ambitious treatment goals, such as complete skin clearance, are within reach with today’s new therapies. To support patients in setting and achieving their personal treatment goals, clinicians should consider individual patient’s comorbidities, as well as their treatment goals, and their engagement in their own treatment. In the future, personalised psoriasis treatment must balance patient’s clinical features and individual priorities with the efficacy and safety of the available therapies, as determined by clinical trials and real-world experience.







