Meeting Summary
This expert masterclass, supported by an independent grant from OM/Vifor Pharma, brought together physicians specialising in vascular surgery, gynaecology, and dermatology from Pakistan, Egypt, Turkey, Lebanon, and Germany to discuss the current management of chronic venous disease (CVD) and haemorrhoidal disease. The meeting included plenary lectures and interactive case study discussions, allowing delegates and presenters to take part in high-level discussions of pressing issues in the field.
CHRONIC VENOUS DISEASE: EPIDEMIOLOGY, RISK FACTORS, PATHOPHYSIOLOGY, AND DIAGNOSIS
Epidemiology
CVD is a very concerning condition which creates a significant burden on healthcare provision and wider society. Varicose veins are often the first sign of CVD. However, progression often over several years to chronic venous insufficiency (CVI), defined by the presence of oedema and a Clinical Etiologic Anatomic Pathophysiological (CEAP) clinical class of ≥C3, represents a more significant problem for healthcare providers and patients (Table 1). In addition, post-thrombotic syndrome also contributes to the burden of venous disease with a similar, though more severe, symptom profile beginning with feelings of heaviness and progressing to pain, oedema, and skin changes.
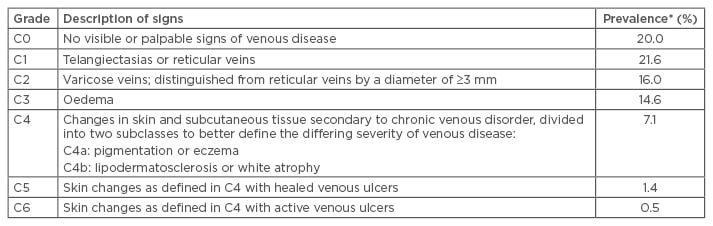
Table 1: Revised Clinical Etiologic Anatomic Pathophysiological classification and worldwide prevalence of chronic venous disease.
Adapted from Eklöf et al., 2004.3 *Data from Rabe et al. 2012.1
Data from the international screening and awareness-raising Vein Consult Program show that the early stages of CVD predominate.1 Varicose veins and oedema affect around 15% of the population worldwide (Table 1). Severe disease, characterised by active or healed venous ulcers, affects approximately 2% of the world’s population (Table 1), with a significant concomitant burden for healthcare systems and patients.2 More recent CEAP-class based studies, conducted in Europe and the USA, put severe disease from C4−C6 at approximately 5% while C2−C3 prevalence is approximately 25%, making moderate-to-severe CVD a very frequent pathology. These figures appear to remain relatively constant on a country by country basis, including across the Middle East and South Asia.
The prevalence of CVI increases dramatically with age, with venous ulcers affecting 20.7% of those >80 years of age compared with 0.3% of 41−50-year-olds.4 CVD is often considered to be more common in women, however both sexes are substantially affected. While the incidence of varicose veins is higher in females than males (13.9–46.3% of females and 11.4–29.3% of males based on 50,974 persons from Europe and the USA),5-10 the prevalence is similar in men, and in women who have never been pregnant. The influence of sex on less severe forms of venous disease is unclear. Furthermore, in the Edinburgh Vein study, varicose veins were more common among male subjects in the general population. Data from the Bonn Vein Study indicate that women with CVD are more likely to report having experienced symptoms occurring over the previous 4 weeks than men with CVD. These differences can be up to 2-fold for symptoms such as heaviness (23.8% versus 11.1%, in women and men, respectively) and pain associated with standing (24.2% versus 14.4% in women and men, respectively). This may be one of the reasons why men with varicose veins seldom seek treatment and present at later stages of CVI.
CVD is a major socioeconomic burden worldwide. In Western Europe, 2% (€900 million) of the annual healthcare budget is spent on chronic venous conditions, the equivalent of 2.5 billion in the USA. This represents more of a budgetary impact than arterial disease. Venous ulcers contribute to $2 million lost work days annually in the USA; CVD in general results in 4 million lost work days annually in France. The annual cost for loss of work days varies from €270 million in Germany, to €320 million in France, and 3 billion per year in the USA.11,12
Risk Factors
The risk factors for varicose veins are non- modifiable and include age, genetics, and female sex/pregnancy. However, CVI has both modifiable and non-modifiable risk factors, which include age, obesity, hypertension, and prolonged sitting or standing. Older age is the most important risk factor for both varicose veins and CVI. In the large cross-sectional San Diego Population Study, the odds ratio (OR) for older age was 2.42 for varicose veins and 4.85 for CVI.5,10 In the large prospective Bonn Vein Study, the OR for varicose veins in 70–79 year olds was 15.9.9,10,13 Obesity is the most significant modifiable CVI risk factor, affecting between three-quarters and two-thirds of patients. Late stage CVI, characterised by ulceration, is extremely rare in non-obese patients. The presence of modifiable risk factors in CVI offers the possibility to institute prophylactic strategies.
Pathophysiology
The major characteristics of CVD pathophysiology are reflux, caused principally by valvular incompetence and obstruction. Obstruction and reflux may exist alone or in combination and are exacerbated by muscle-pump dysfunction or inactivity. These factors result in elevated ambulatory venous pressure leading to ambulatory venous hypertension. Increased venous pressure and reduced blood flow caused by the above mechanisms are the major drivers of the downstream pathological features of CVD.
Up until the turn of the century, the primary theory of varicose veins pathophysiology was based on a descending phenotype of disease progression. The ‘descending theory’ postulates initiation of disease cranially, at the level of the saphenofemoral or saphenopopliteal junctions, followed by extension to the main trunks and the superficial tributaries.11 However, over the past 10–15 years the ‘ascending theory’, suggesting a multifocal origin beginning with the tributaries and moving to the trunks and then junctions, has gained traction.10,14
Inflammation has a key role in valve failure and vein wall remodelling, both of which contribute to reflux and hypertension. Increased venous hydrostatic pressure and low blood flow activate inflammatory gene expression in the vascular endothelium. This leads to leukocyte rolling, adhesion, and migration. The above effects contribute to inflammation and free-radical formation which in turn induce apoptosis and tissue necrosis. Thus, the venous wall and valve damage found in primary venous disease becomes self-reinforcing.15-17
Similarly, at the level of the microvasculature, reduced blood flow and increased blood pressure leads to activation of cell-cell interactions, inducing white-cell trapping, which in turn leads to the release of inflammatory mediators. Inflammation leads to the opening of the endothelial barrier and venular endothelial-cell leakage. When fluid leaving the capillary (capillary filtration) exceeds lymphatic flow the result is interstitial oedema. The fluid and immunologically active cells entering the surrounding tissue lead to further release of inflammatory mediators exacerbating oedema and causing pain and discomfort. Since the pathological mechanisms directly resulting in oedema occur within the microcirculation, this is the major target for pharmacological interventions against CVI.
Diagnosis
The presence of feelings of heaviness, tiredness, and swelling is a diagnostic feature of CVD and can distinguish CVD from other sources of leg pain such as arterial occlusive disease. In addition, pain which increases throughout the day, in warm temperatures, and predominantly with standing rather than walking are indicative of a venous pathology. Diagnostic techniques can be separated into three levels: 1) history and examination; 2) non-invasive imaging; and 3) complex or invasive imaging. The European Society of Vascular Surgery (ESVS) recommends history-taking and physical examination with an evidence level of 1C.10
The CEAP system (Table 1) is generally accepted as the best of the available diagnostic documentation systems (1B). A number of supplementary clinical scoring systems also exist. The Venous Clinical Severity Score appears to be underutilised in some clinical settings, despite its positive impact on assessment of disease severity and patient wellbeing (2aB).10 The Venous Segmental Disease Score (VSDS) and Venous Disability Score (VDS) also represent useful diagnostic tools (2aB).10 Routine use of these additional tests is becoming more frequent and should be encouraged.
Assessment of patient quality of life (QoL) should be incorporated into clinical examination. QoL correlates strongly with disease severity and treatment-induced improvements, and is increasingly viewed as the most important outcome measure when assessing novel therapies.10 A number of QoL measures, both general and venous-disease specific, are available and are recommended by the ESVS (2aB). Both physical and mental components of the generic SF-30 questionnaire can detect the impact of CVD on QoL. Data indicate that Stage C3 has a similar impact to diabetes or cancer while Stage C5–C6 has a similar impact to heart failure.18 The Aberdeen Varicose Vein Quality of Life (AVVQ) questionnaire, alongside the Chronic Venous Insufficiency Questionnaire (CIVIQ), or Venous Insufficiency Epidemiological and Economic study questionnaire, represent good-quality disease-specific measures for assessing response to treatment (2aB).10
In terms of non-invasive diagnostic imaging, duplex ultrasonography (DUS) is now the gold standard across the world (1A).11 DUS is sufficient to investigate the deep venous system in the majority of cases and should be used before considering phlebography, computed tomography venography (CTV), or magnetic resonance venography (MRV) for difficult to visualise pelvic or abdominal veins (1C).11 With the adoption of DUS, handheld Doppler is now considered obsolete.10
In cases of deep abdominal or pelvic veins which cannot be visualised with DUS, CTV and MRV are frequently used in well-resourced centres and can now achieve three-dimensional images (1C).10 Reliable diagnosis of abdominal pathologies including Nutcracker syndrome, May–Thurner syndrome, and pelvic varicocele can now be achieved with these techniques.10 Invasive techniques such as phlebography are recommended where other tools are inconclusive (2bB).10
MANAGEMENT OF CHRONIC VENOUS DISEASE
Treatment of CVI aims to both improve venous function and improve the signs and symptoms of CVI. Addressing signs as symptoms is the most important goal of treatment from the patient perspective, however, this is not always concomitant with improved venous function. For example, the frequent recurrence of venous ulcers following invasive treatment is driven by continued microcirculatory pathology, which is not affected by addressing venous reflux.
Management should also address the progressive nature of CVI and act as a prophylactic against aggravation and progression.19 Treatment comprises both conservative and invasive therapeutic options. Conservative treatments include physical treatment, such as engaging in increased movement or sports, compression treatment, and pharmacological therapy. Invasive treatments include surgery of superficial and deep veins, including percutaneous phlebectomy using hooks and stripping techniques. New less invasive treatment options include foam sclerotherapy, endovenous thermal ablation, and radiofrequency ablation techniques.20-22 These techniques offer good mid-term results and have comparable efficacy to stripping, though vein diameter should be considered when making treatment decisions as larger varicose veins may not be as amenable to these less invasive techniques.
Compression Therapy
Compression therapy is the foundation of therapeutic management of CVI. As well as gross physical effects on oedema, compression reduces inflammation by tightening the gaps between vascular endothelial cells and by reducing cell-cell interactions. Intermittent venous hypertension is also reduced and capillary and venular-flow velocity enhanced, addressing primary drivers of CVI pathology. Compression improves haemodynamics through promotion of the venous pump and reduced venous reflux. While oedema formation is ameliorated through improvements in microcirculation and promotion of lymphatic drainage. Oedema reduction results in improvement of symptoms with the added advantage that these effects are immediate.
Compression treatment takes on a number of forms including bandages, stockings, and intermittent pneumatic compression, which is emerging as a potentially exciting new method of thromboprophylaxis. In CVD, indications for compression stockings vary according to their pressure. Low-pressure (10–20 mmHg) stockings are indicated for treatment of CEAP classification C0–C1 with the aim of preventing swelling, heavy legs, tenderness, and pain. Symptom prevention, at the C2 stage, and oedema reduction, at the C3 stage, requires higher pressure stockings of 20–30 mmHg. Stockings with a pressure of 30–40 mmHg are indicated for ulcer prevention (C4) and ulcer-recurrence prevention (C5). While ulcer healing and pain relief for patients with C6 CVI requires the use of compression-stocking kits with pressures of ~40 mmHg.
The consensus document from 2008, which will be updated shortly, reveals a number of gaps in the evidence base for compression therapy.23 Currently, there are few recommendations with the highest 1A evidence level. Prevention of venous thromboembolism using low-pressure compression and the prevention of C6 ulcers using compression bandages are level 1A recommendations; however, the use of high-pressure compression stockings for the prevention of post-thrombotic syndrome, currently 1A, is likely to be downgraded on the strength of data from the SOX trial.24 A significant increase in data available indicating that post-surgical compression for pain relief is only efficacious during the first week post-surgery is also likely to affect recommendations in the new consensus document.
Venoactive Drugs
The actions of venoactive drugs are very similar to those of compression therapy. Venoactive drugs reduce inflammation of the venous wall, reduce oedema formation and the development of skin changes, and protect endothelial cells from contraction. Venoactive drugs are a heterogeneous group, many of which are derived from plant extracts (Table 2). Their evidence base is similarly heterogeneous with a significant number of therapies having little or no peer-reviewed evidence available.
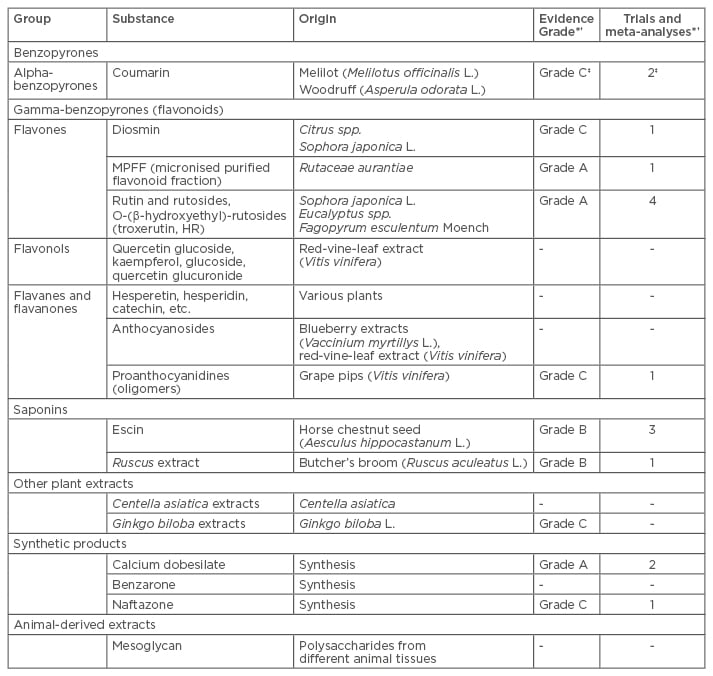
Table 2: Summary of venoactive drugs.25,26
†Only trials assessing symptoms were considered when assessing evidence grade. ‡Evidence from combined coumarin-troxerutin therapy.
MPFF: micronised purified flavonoid fraction.
Adapted from Ramelet et al., 2005.25 *Based on data from Nicholaides et al., 2008.26
Relief of symptoms is the main objective of pharmacological treatment, and strong evidence exists for an objective effect on oedema and venous symptoms (evidence level in most of the cases between 1 and 2B depending on the substance used). Whether pharmacological therapy can heal CVD remains unclear, though evidence suggests a possible effect on leg-ulcer healing. Similarly, prophylactic use of venoactive drugs has yet to be sufficiently investigated, though CVD pathophysiology and the anti-inflammatory mechanisms of venoactive drugs would suggest that retardation of progression is possible. Data indicate that the use of venoactive drugs is both safe and economic, with digestive side effects the main notable adverse outcomes.25,26
Despite the strong evidence base for an objective effect, some physicians question the clinical significance of the oedema reduction achieved by venoactive drugs. Data from a 2006 review show that the majority of reductions range from 40–50 mL, which does not appear to be clinically relevant given the volume of the calf as a whole.27 However, this comparison is based on a number of erroneous assumptions. Firstly, water displacement volumetry, the primary method used to assess oedema, principally measures the foot and ankle area, representing around 2,500–3,000 mL. Though this is still a large volume in comparison to reductions achieved with venoactive drugs, venous oedema mainly occurs in the cutis and subcutis, representing approximately 20% of this volume (600 mL). Furthermore, only around one-third of this fatty tissue is made up of water, reducing the volume of tissue exposed to oedema to approximately 200 mL. With these adjustments made the effect achieved by venoactive drugs in fact represents a reduction in volume of approximately 25%.
Another controversy relates to the place of venoactive drugs in therapy and whether they can replace compression therapy, surgery, or both. The majority of venoactive drugs require a 3−4-week run-in phase before achieving oedema-reducing efficacy, making them unsuitable to replace compression therapy in many situations where an immediate reduction in oedema is required. Similarly, unlike surgical interventions, venoactive drugs cannot restore varicose veins or resolve obstruction. In fact, pharmacological interventions represent an excellent synergistic therapy alongside both compression therapy and surgical interventions, rather than a potential replacement.
CALCIUM DOBESILATE: CLINICAL EFFICACY IN THE TREATMENT OF SYMPTOMATIC CHRONIC VENOUS DISEASE
Calcium dobesilate affects a number of pathophysiological mechanisms to improve the symptoms of CVI. These mechanisms can be broadly categorised as capillary and vein-wall effects, blood-component effects, and lymphatic-circulation effects.28,29
The effects of calcium dobesilate on the capillary and vein wall include: reduction of reactive oxygen species, reduction in apoptotic factors, amelioration of endothelial dysfunction, inhibition of leukocyte adhesion to the endothelium, and reduction of capillary-wall permeability via anti-vascular endothelial growth factor activity and inhibition of alterations to cell-junction proteins.28-35 These combined mechanisms act to improve capillary and vein wall function and integrity, reducing blood and fluid extravasation and, thus, oedema formation.
Calcium dobesilate acts on blood components to reduce erythrocyte fragility and inhibit platelet aggregation, in addition to reducing plasma viscosity.36,37 These effects may in turn reduce thrombosis in the microcirculation and the associated symptom, white atrophy.
Finally, improved capture of fluid and macromolecules from the extracellular space and accelerated lymph flow acts to normalise lymph physiology and improve symptoms.38,39 The understanding of the importance of this mechanism has increased over the past 10 years as the weight of evidence now suggests that ≥90% of the fluid contributing to oedema is reabsorbed by the lymph system.40-42
Clinical Effects of Calcium Dobesilate
Clinical data indicate calcium dobesilate is efficacious in reducing the symptoms and signs of CVI. A 2001 meta-analysis by Espinosa and Giannone43 synthesised the results of four randomised, double-blind, placebo-controlled clinical studies where patients received 1.5 g of calcium dobesilate per day or placebo for at least 4 weeks (N=324). Calcium dobesilate achieved significant improvements in the most frequent subjective symptoms compared with placebo.
A 2004 randomised, double-blind, placebo-controlled trial of calcium dobesilate (1.5 g/day for 4 weeks) in outpatients (N=253) with CVI (CEAP: C3−4) investigated effects on oedema and leg volume. Despite the need for a 3−4-week run-in period to begin reducing oedema, there was a significant (p=0.0109) 48% decrease in leg volume in moderate CVI after 4 weeks of calcium dobesilate treatment (Figure 1a). Following withdrawal of treatment the reduction in oedema partially reversed, although subsequent trials suggest a long-term effect can be achieved with calcium dobesilate. In patients with more severe CVI (patients with previous treatment, CEAP Stage C4, or CVI duration >12 years) a 77% reduction was achieved (Figure 1b).44 A more recent meta-analysis by Ciapponi et al.45 on three calcium dobesilate trials (N=608) found similar results including a greater improvement in pain, heaviness, and ankle swelling in patients with severe stages of the disease compared with those with mild CVI.

Figure 1: Changes in the leg volume during therapy with calcium dobesilate or placebo in patients with A) moderate or B) more severe chronic venous insufficiency.44
Copyright © 2016 by European Medical Journal. Reprinted by Permission of SAGE Publications, Ltd.
In order to conform with updated guidelines, the effect of calcium dobesilate on calf volume and pain was investigated over 8 weeks in a double-blind, placebo-controlled multicentre trial (N=256). Patients had symptomatic CVI and pitting oedema (C3–C5) and at least one symptom such as discomfort or pain. Calf volume was measured with the circumference method, rather than water displacement volumetry. The volume of the lower calf diminished in the calcium dobesilate group at the end of the active treatment period by 64.7 mL compared with 0.8 mL in the placebo group (p=0.0002), independent of compression-stocking use. Pain was reduced in both groups but the reduction was significantly more pronounced in the calcium dobesilate group compared with placebo (p=0.0071).46
Due to the preference of some regulatory authorities for data from water displacement volumetry, a similar study was carried out using this outcome measure to assess oedema. This randomised, double-blind, placebo-controlled study assessed calcium dobesilate at 1.5 g/day over 12 weeks in patients with CVI at Stage C3–C4 (N=351). The primary endpoint of relative volume change in the most pathological leg from baseline to Week 12 was not achieved in this study (-0.6% versus -0.3%; p=0.09), likely due to a number of protocol violations related to inadequacies in the accuracy of water displacement volumetry out with the laboratory setting. However, at a 24-week follow-up, patients previously treated with calcium dobesilate showed a significant change in both relative (-1.01% versus -0.08%; p=0.002) and absolute volume (-25.7 mL versus -1.1 mL; p=0.002) versus placebo. These data suggest a putative long-term effect of calcium dobesilate which warrants further investigation and may affect future guidelines regarding the optimal time course of calcium dobesilate therapy.47
The importance of QoL data in CVI has been previously highlighted. In the above study, the CIVIQ QoL subscore assessing pain or discomfort in ankles or legs during the past 4 weeks was significantly improved by Week 12 in the calcium dobesilate group (p=0.03) compared with baseline, though this difference was not significant compared with placebo.47 Long-term QoL data, from a 2008 study by Martínez-Zapata et al.,48 showed a significant difference in favour of calcium dobesilate compared with placebo at 12 months (p=0.02). In this study, as in the 2016 study by Rabe et al.,47 QoL improved in both the calcium dobesilate and placebo group up to Month 3. However, over the longer-term QoL continued to improve in the calcium dobesilate group, while in the placebo group it deteriorated to a point below baseline values.48
Consensus and Guideline Documents
The Siena Consensus document represents one of the earliest guidelines for CVI treatment. Recommendations in this document were based on data pertaining to symptom improvement rather than clinical signs such as oedema.25 The Siena document graded the use of venoactive drugs for CVI with evidence level A, and this was confirmed in the International Guideline On Management of CVD, published in 2008, which recommended calcium dobesilate specifically with an evidence level of A based on two trials/meta-analyses (Table 1).25,26 In terms of other venoactive drugs, micronised purified flavonoid fraction (MPFF) also had a Grade A recommendation while the remaining pharmacological therapies were graded C or B, and were not recommended as first-line treatments.26 The 2014 update of this guideline recommended MPFF at Grade 1B versus 2B for calcium dobesilate with both drugs having the same level of clinical evidence (B).49 In a more technical evaluation of the data from the 2014 Austrian Health Technology Assessment report, only calcium dobesilate had a moderate level of evidence from placebo-controlled trials with the remaining therapies having only a low strength of evidence.
The most rigorous and up-to-date evidence currently available for venoactive drugs comes from the 2016 Cochrane review on phlebotonics for CVI. Sixty-six randomised controlled trials were assessed, and 53 trials (N=6,013) provided quantifiable data. The majority of trials were on rutosides (28), followed by hidrosmine and diosmine (10), and calcium dobesilate (9). Most studies were flawed with only four presenting a low risk of bias. Of these high-quality studies, three assessed calcium dobesilate and one assessed coumarin/troxerutin dual therapy. The authors concluded that moderate-quality evidence showed that phlebotonics have beneficial effects on oedema and on some signs and symptoms related to CVI when compared with placebo. There was an increased risk of gastrointestinal adverse events compared with placebo. The meta-analysis found no difference in terms of ulcer healing, though very few studies looked into this. Concluding, the authors also emphasised the need for further high-quality randomised controlled trials to improve the evidence base for phlebotonics in CVI.50
IMPACT OF CHRONIC VENOUS DISEASE ON DIABETIC LIMB SALVAGE
Leg ulcers can be grouped with those of a mainly venous origin (Group I: 75%), those of a mixed venous and arterial origin (Group II: 14%), and those contributed to by both CVI and diabetes (Group III: 9%), which more often affect the foot. Group II ulcers are often misdiagnosed resulting in incomplete or erroneous therapeutic strategies. Those ulcers of a mixed diabetic and venous origin (Group III) represent a significant treatment challenge, often failing to heal, leading to limb amputation.
Forty percent of diabetic foot lesions are associated with CVI. The combined features of diabetes and CVI create treatment-resistant ulcers which increase the risk of limb loss. When encountering a patient with a diabetic ulcer and CVI, general examination followed by regional and finally local examination should be carried out. Underlying general aetiological features which may be contributing to ulcer formation, such as rheumatoid arthritis, systemic lupus, and uncontrolled diabetes, must be taken into account. Next, regional aetiological features such as ischaemia and CVI which may retard the healing process should be noted. Finally, local disease features including arterial ischaemia, venous hypertension, and lymph oedema should be accounted for.
Experience from large centres which receive high numbers of referrals for limb amputation due to diabetic foot lesions suggests that a lack of dedicated foot-care teams combined with narrow speciality-focussed, rather than pathology-focussed treatment strategies contributes to limb loss in diabetic patients. For example, a vascular surgeon may, upon finding no pulse in a foot affected by diabetic lesions, label this a vascular case. Investigation and correction of the source of this issue, such as a superficial femoral occlusion, may not lead to healing of the diabetic lesion as issues such as poor blood quality have been missed in the narrow specialist-driven focus on vascular disease. These problems could be overcome through oversight by a multidisciplinary team; however, in the real-world clinical environment the availability of such teams is vanishingly small, even in highly developed healthcare systems such as that of the USA.
A recently published paper aimed to develop a strategy to overcome these issues using data from an internal hospital-based analysis carried out in Cairo University’s Hospitals. The analysis identified six modifiable aetiological factors in patients with diabetic foot lesions, who were referred for major amputation. The impact of targeted treatment aimed at these modifiable factors (blood quality [haemoglobin <9 g/dL and/or serum albumin <3 g/dL], blood quantity, bone lesions, associated diseases, tissue loss, and previous local foot surgery) was then analysed prospectively over 5 years in a population of 4,102 patients.51
In this study, one of the six identified aetiological factors was present in all the referred cases, and in the majority of cases there were two or more factors present (78%). The most common aetiological factor was blood quality (66%) while the least common was tissue loss (8%). The presence of associated disease, which included CVI, was a factor in 28% of cases.51
Use of an aetiologically-driven treatment paradigm resulted in limb salvage in the majority of patients referred for amputation. Cases involving associated diseases, such as CVI, were the most successfully treated with only 16% proceeding to major amputation, while those associated with tissue loss and previous foot surgery were the least successful with 35% and 52%, respectively, necessitating amputation (Figure 2). Cases with previous foot surgery were thought to be the most problematic due to a history of progressive local tissue loss caused by localised interventions undertaken without adequate attempts to address the underlying aetiological cause.51
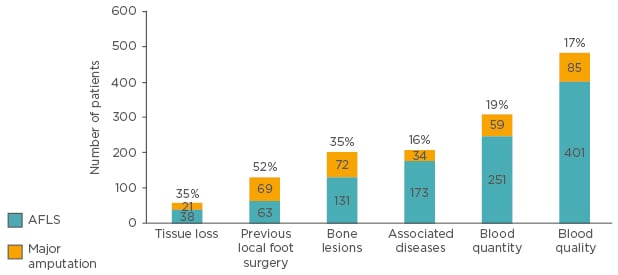
Figure 2: Major amputation and amputation-free limb salvage following aetiological key-based treatment in diabetic ulcer patients referred for major amputation.
The authors’ recommendation was to apply the diabetic foot protocol to identify, detect, and correct all aetiological features as part of a strategic management plan. Use of a colour-coded key displaying the six aetiological factors as the cover sheet of the patient’s file was also recommended. Finally, categorisation of the patient by respective physician speciality was strongly discouraged because of the effects on downstream discovery of aetiological features.
ALFS: amputation free limb salvage.
SUMMARY OF CASE STUDIES
Case Study 1
A 79-year-old female patient presented with a venous leg ulcer. The patient had a small saphenous varicose vein on her right leg and complained of feelings of heaviness, pain, and swelling. Ulcer healing was achieved following endovenous laser therapy and compression. However, moderate pain, feelings of heaviness, and slight oedema remained. Following 6 weeks of 1.5 g/day calcium dobesilate, pain, feelings of heaviness, and oedema had all resolved.
Case Study 2
A 49-year-old male of average weight and height presented due to a history of recurrent left calf popliteal deep vein thrombosis (DVT), consisting of 3 attacks over the previous 4 years. Route lab results were normal, and the patient was negative for protein C and S, and Factor V Leidin mutation. The patient was using routine oral anticoagulant and had an international normalised ratio of 2.2–3.0. The patient’s acute presentation was a rapid progressive left-limb oedema and foot cyanosis, without groin or scrotal oedema. The clinical presentation was that of phlegmasia dolens and DUS showed a left iliofemoral DVT.
Thrombolytic therapy was indicated due to the phlegmasia dolens and foot cyanosis. An inferior vena cava filter was inserted through the right femoral vein. Then a perfusion catheter was inserted by the popliteal route, ascending through the popliteal vein to the left iliofemoral vein, allowing tissue plasminogen activator therapy for 24 hours which resulted in rapid resolution of the oedema. However, following treatment, venography revealed the underlying aetiology to be left common iliac vein compression syndrome by the right common iliac artery. Following dilation, a stent was inserted and the patient was allowed to recover for 2 days under observation which resulted in resolution of the issue with no recurrence for >17 years.
Case Study 3
A 65-year-old man with no history of diabetes or hypertension presented with a swollen left leg, which had been present for the past 10 months, and a small swelling in his left groin. DUS revealed an acute thrombosis in the femoral popliteal vein, a floating non-adherent common femoral vein thrombus, and a compressed narrowed left iliac vein caused by a soft tissue mass. Magnetic resonance imaging (MRI) showed an amalgamated group of left external iliac lymph nodes cuffing the external and internal iliac vessels. The arteries were enhancing while the veins were obliterated, the left external iliac vein by the mass of a lymphadenopathy and the left external iliac vein by the amalgamated group of left external iliac lymph nodes.
Biopsy revealed non-Hodgkin lymphoma; the lymphoma was follicular in nature and characterised by macroscopic follicularly observed in cross sections of the lymph node. Treatment of this tumour type with rituximab is known to produce a high response rate with an extremely favourable toxicity profile. Reductions in tumour size of 90% can be expected in 5–9 months with a remission-rate ranging from 75–90%.52
The attending physician was faced with three possible management plans for the initiation of treatment, address: the tumour, the floating thrombus, or the ilio-femoral DVT. Due to the possibility of rapid tumour shrinkage with rituximab which could result in freeing of the thrombus and a subsequent risk of pulmonary embolism, the physician inserted a retrievable inferior vena cava filter to deliver iliofemoral catheter directed thrombolytic therapy. Therapy with rituximab was started following catheter placement and calcium dobesilate therapy initiated to deal with the potential for venous hypertension. After 2 weeks, the groin swelling and oedema was resolved.
SUMMARY
CVD creates a significant burden on healthcare provision and wider society. CVD pathophysiology is driven by increased venous pressure, reflux, and/or obstruction, resulting in venous hypertension and reduced blood flow. These factors induce inflammation in the microcirculature and subsequent oedema. The primary common goal of all treatment methods is the improvement of clinical signs, symptoms, and patient QoL. Technical common goals, including ablation of reflux and improvement of venous function, are secondary to symptom relief in the eyes of both patients and modern clinicians. Both invasive techniques and compression act to achieve the combined goals of symptom reduction and resolution of pathological mechanisms. Pharmacological interventions represent a complimentary therapeutic option acting on both symptoms and mechanisms.
Calcium dobesilate has a unique multi-target mode of action which both preserves microvascular integrity and improves microvascular circulation. Calcium dobesilate has specific effects on capillary permeability and oedema reduction; however, its effects are broader and include anti-inflammatory mechanisms of action. Calcium dobesilate effectively improves CVI symptoms and is well tolerated with a low incidence of minor reversible digestive disorders. Finally, and most importantly, calcium dobesilate improves the QoL of patients.







