Interviewees: Sascha Gerdes,1 Julia-Tatjana Maul2
1. Center for Inflammatory Skin Diseases, Department of Dermatology, Venereology and Allergology, University Medical Center Schleswig-Holstein, Kiel, Germany
2. Department of Dermatology and Venereology and Allergy, University Hospital Zurich, Zurich, Switzerland
Disclosure: Dr Gerdes has been an advisor, and/or received speakers’ honoraria, and/or received grants, and/or participated in clinical trials for AbbVie, Affibody AB, Akari Therapeutics, Almirall-Hermal, Amgen, AnaptysBio, AstraZeneca AB, Baxalta, Bayer HealthCare, Biogen Idec, Bioskin, Boehringer Ingelheim, Celgene, Centocor, Dermira, Eli Lilly, Foamix, Forward Pharma, Galderma, Hexal AG, Incyte, Isotechnika Pharma, Janssen-Cilag, Johnson & Johnson, Kymab, LEO Pharma, Medac, Merck Serono, Mitsubishi Tanabe Pharma, Mölnlycke Health Care, MSD, neubourg skin care GmbH, Novartis, Pfizer, Polichem SA, Principia Biopharma, Regeneron Pharmaceutical, Sandoz Biopharmaceuticals, Sanofi-Aventis, Schering-Plough, Sienna Biopharmaceuticals, Takeda, Teva, Trevi Therapeutics, UCB Pharma, and Vascular Biogenics. Dr Maul is an employee of the University Hospital Zurich and holds a ‘Filling the Gap’ scholarship; has served as an advisor, and/or received speaking fees, and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, LEO Pharma, Janssen-Cilag, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, and UCB.
Acknowledgements: Writing assistance was provided by Dr Eleanor Roberts, Beeline Science Communications, Ltd, London, UK.
Disclaimer: The views and opinions are those of the authors and not necessarily those of Almirall.
Support: The publication of this article was funded by Almirall.
Citation: EMJ Dermatol. 2021;9[Suppl 3]:2-8.
Meeting Summary
Plaque psoriasis is a chronic inflammatory condition that can occur as a result of aberrant signalling between the immune system and epidermal cells. As the cytokine IL-23 is a ‘master controller’ of these interactions, inhibition can downregulate the signalling cascade, which includes differentiation of pathogenic Th17 cells and growth of keratinocytes. Because of its mechanism of action, treatment with tildrakizumab, which targets the p19 subunit of IL-23, has been shown to lead to long-lasting disease control over a period of up to 5 years. Post hoc analysis of clinical trials of tildrakizumab as a maintenance treatment showed that patients who initially had a good response after 28 weeks could maintain this over 5 years with a safety profile similar to other IL-23p19 inhibitors. As discussed in this interview with two dermatologists who have had experience of tildrakizumab use in their daily practice, Dr Gerdes and Dr Maul, such data are important as it gives both clinicians and patients confidence in using tildrakizumab over an extended time period. The clinicians discussed how response to a psoriasis therapy can be tracked using the Psoriasis Area and Severity Index (PASI) score, which they would like to see below 3 for most patients, but that this must be integrated with subjective responses and used to tailor individualised treatment plan needs and goals.
INTRODUCTION
The indicative scaly, erythematous plaques of plaque psoriasis arise because of abnormal interactions between immune system cells and epidermal keratinocytes.1 Available therapies for psoriasis have moved from generic anti-inflammatory drugs, such as topical corticosteroids, and nonspecific systemic immunosuppressants, such as methotrexate or cyclosporine, to now include monoclonal antibodies that specifically target key inflammatory cytokines involved in psoriasis development and exacerbation.1 Advantages of these newer ‘biologic’ therapies not only include better disease control but also relatively good safety profiles, allowing for use over long periods of time.1,2
At the European Academy of Dermatology and Venereology (EADV) Virtual Congress 2020, data on the efficacy and safety of the biologic tildrakizumab over 5 years of administration in adults with moderate-to-severe plaque psoriasis were presented.3-8 EMJ spoke to dermatologists Dr Maul and Dr Gerdes to gain an understanding of IL-23 inhibitors’ mechanism of action, treatment goals for psoriasis, and how the long-term efficacy and safety data from the tildrakizumab clinical trials reSURFACE 1 and 2 fit into these objectives.
WHAT IS THE IMPORTANCE OF IL-23 IN THE DEVELOPMENT OF PSORIATIC PLAQUES?
Differentiated Th17 cells were identified over a decade ago as playing a major role in psoriasis.9 Dr Maul explained: “IL-23 has been described as the master cytokine regulating differentiation of pathogenic Th17 cells that in turn produce IL-17, the cytokine responsible for abnormal keratinocyte proliferation.” IL-23 is mostly derived from dendritic cells present in the skin, Dr Gerdes explained. Its release can further be stimulated by activated keratinocytes, leading to more IL-23 production and stimulation of both Th17 cells directly and other T cells important for IL-17 secretion.9
HOW DO IL-23-TARGETING THERAPIES WORK TO CONTROL PSORIASIS?
“By targeting IL-23,” said Dr Gerdes, “you are cutting off this cascade at the very top and not later downstream, such as by inhibiting IL-17 itself.” Due to this long-lasting downregulation of pathogenic Th17 cells, IL-23-targeting therapies are, according to Dr Maul, “very sustainable,” and translate into “long-lasting efficacy and benefit for patients.” Dr Gerdes continued: “A good metaphor is that IL-23 is the teacher, teaching a lot of students (representing downstream cytokines and cellular targets). If you take out the students, the teacher is still there, recruiting more students, but if you take away the teacher, it has much more impact because you need a new teacher if you want to educate any more students.”
Tildrakizumab specifically targets IL-23 at the p19 subunit. It is delivered via subcutaneous injection at maintenance intervals every 12 weeks.10,11 Dr Gerdes highlighted that by inhibiting IL-23 only at the p19 subunit, tildrakizumab, and other IL-23p19 inhibitors such as guselkumab and risankizumab, inhibit this cytokine alone. This contrasts with the biologic ustekinumab, which targets the p40 IL-23 subunit, shared with, and thus also inhibiting, the cytokine IL-12.12
This is reflected in a recent post hoc analysis of clinical trials for tildrakizumab that showed, of those who had an initial response to tildrakizumab ≥75% PASI score improvement, the median time to relapse (loss of PASI 75 response) was 20 weeks with 100 mg tildrakizumab and 25 weeks with 200 mg tildrakizumab (i.e., 32–37 weeks after the last tildrakizumab dose).13
WHAT IS THE CLINICAL RELEVANCE OF THE PASI SCORE USED IN THE reSURFACE 1 AND 2 TRIALS TO TRACK TILDRAKIZUMAB RESPONSE?
PASI is used to score and track psoriatic plaques by assessing both degree of severity and amount of skin surface involved over four different body regions. With a score between 0 and 72,14 PASI can be tracked as either the absolute score or by score change from one assessment to the next. Whether to use absolute PASI or change in PASI score is a topic of great debate in research and clinical practice, Dr Gerdes reported.
In randomised clinical trials, explained Dr Maul, PASI is the gold standard for assessing treatment response, thus allowing for comparison between studies. Historically, percentage change in PASI score has been the main trial objective, with many deeming a specific percentage reduction, indicating improvement of the PASI score, as a ‘response’; for instance, by 75% (PASI 75) or 90% (PASI 90).10,11 However, Dr Maul stated: “The absolute PASI is becoming increasingly important because we would like our patients to reach a PASI less than 3, independent of their initial [baseline] PASI score.” She explained that it is not just because “the absolute PASI score is very relevant and easier to compare than using the PASI 75 or 90 response,” but also because “when you’re starting with a lower PASI, it’s a lot harder to have a PASI 90 response than when you’re starting with a very high PASI.” Conversely, she explained, “when starting with a very high PASI, having a PASI 90 response might still not be an absolute PASI of 3.”
In one of the 5-year post hoc analyses of the tildrakizumab reSURFACE 1 and 2 trials, the cohort included those who achieved an absolute PASI score <3 at Week 28.3 This retention of a predetermined absolute PASI is, according to Dr Gerdes, “a very good tool to look at efficacy over time. If you are looking at an absolute PASI below 3, you know every patient who has this over 5 years has good control at any time point. If you are looking at the delta PASI, you need to know what the PASI was at baseline to know if this is a good result or not.”
WHAT ARE THE SHORT- AND LONG-TERM THERAPY GOALS FOR PEOPLE WITH PSORIASIS?
Dr Maul reported that, as expected, short-term goals for patients have included being free of plaques and therefore having clear skin; however, she stressed the importance of ascertaining individual patient requirements as treatment goals may differ according to the person’s wants and needs. “Some patients might be happy with an absolute PASI of 5 but, depending [on other factors], for example on [plaque] location, others may want an absolute PASI of 1 or less.” There are also goal differences to be aware of between male and female and older and younger patients, as highlighted by a recent publication by Dr Maul that assessed patient needs and expectations.15
Reflecting on these individual needs, the dermatologists discussed how several European country guidelines for psoriasis include both objective and subjective parameters.16 “We have to keep in mind,” said Dr Maul, “that not everyone needs to be free of the skin lesions, some patients rather would just like to be free of itch.” She added: “It’s important that we compare PASI to the Dermatology Life Quality Index (DLQI).”17 This ten-point questionnaire represents the impact on a patient’s quality of life, including the symptom pruritus, which, according to Dr Gerdes, is “the strongest connector for life quality besides inflamed skin.” Understanding this is imperative because, he described, “for a very long time we did not, as physicians, recognise that this is such an important point for the patient.” Unlike with skin disorders such as atopic eczema, Dr Gerdes explained that dermatologists do not usually see a lot of scratch marks at lesion sites in the psoriatic population, “but they [patients] are telling us that they experience itch. That is maybe a reason why dermatologists for a long time did not believe that pruritus was a problem for psoriasis patients, but now we have good results from questionnaire studies where patients are really answering this as one of the most important issues.”
“Guidelines for psoriasis treatment include time periods up to 28 weeks when detailing short-term expectations.”18 Though the short-term patient goal is often to see a quick effect, Dr Maul stated: “I try to explain that having a chronic disease and learning to live with it is a process that can take a long time even when on treatment. As such, the speed of onset of a treatment is often not the deciding factor, but rather long-term safety and efficacy.”
Long-term goals are to keep the absolute PASI low and to have a good quality of life. This latter point includes, explained Dr Maul, that “a treatment is simple and easy to use. For example, with the new IL-23 inhibitors, patients do not need to inject as often as they needed to with most of the anti-IL-17 inhibitors, or before that with anti-TNF inhibitors.”
AT WHAT POINT WOULD YOU TREAT SOMEONE WITH PSORIASIS WITH AN IL-23-TARGETING THERAPY?
Both panellists agreed that currently, they would still consider a conventional therapy for first-line treatment, especially in those with moderate psoriasis. If this did not work, or if the patient experienced side effects/tolerance issues, they would consider a biologic next. This included either a TNF, IL-17, or IL-23 inhibitor; “which you use depends on different things,” explained Dr Gerdes. “If patients are suffering from another disease, using other medications, or have other chronic inflammatory diseases, then you need to judge which strategy is best. If it only comes to psoriasis vulgaris and if I could use a biologic, I would tend to use an IL-23 inhibitor.”
Where each country’s healthcare system places IL-23 inhibitors on the treatment pathway must be considered. Dr Maul and Dr Gerdes discussed their interest in whether first-line use of an IL-23 inhibitor in a patient with moderate-to- severe psoriasis can result in memory T-cell regulation and positively impact further disease progression over that person’s lifetime. This could lead to long-term control of psoriasis, beneficial to both the patient and clinician, and is potentially cost-effective because, according to Dr Gerdes, “the most expensive part of therapy is the induction period.”
WHAT ARE YOUR THOUGHTS REGARDING TILDRAKIZUMAB’S EFFICACY OVER 5 YEARS?
The 5-year post hoc efficacy data analysis from the reSURFACE 1 and 2 trials examined study participants receiving 100 mg or 200 mg tildrakizumab throughout the study who at Week 28, in the all-centre dataset analysis, had achieved an absolute PASI <3,3 or in an analysis of the European centres only achieved PASI 75.4 At the 5-year time point in the all-centre analysis, 84.5% of those who received 100 mg and 88.3% of those who received 200 mg still had a PASI <3.3 In the European centre analysis at 5 years, PASI 75 responders receiving 200 mg or 100 mg of tildrakizumab were 93.5% and 91.8% of the study cohort, respectively.
“The data are convincing,” said Dr Gerdes, “they nicely show that patients are well controlled over time in as good a way a study like this can show.” Dr Maul noted: “I feel it’s very important for doctors to tell our patients when we have such good efficacy data over 5 years as this can help the patient’s confidence with a treatment. This in turn can help alleviate a patient’s psychological burden of disease.”
However, as every patient in daily practice is different, both clinicians stressed that it is now important that real-world data is collected. “Here,” said Dr Gerdes, “you are only looking at the patients who were treated with the same dose of tildrakizumab right from baseline to 5 years.” Dr Maul agreed, adding how important it is to “see how tildrakizumab works in real-world conditions with patients with more comorbidities, who are elderly, or who are maybe not as compliant as they should be. Those data will be very important in comparison to the very nice results we see over 5 years.”
Additionally, Dr Gerdes highlighted how ‘real-world’ patients are usually started on a biologic when they have a much lower PASI than that required for clinical trials. “You can only be included in clinical trials if you are above 12 and usually, we see a mean PASI of above 20. However, in the Corrona [Psoriasis] Registry in the USA,19 a very big registry for biologics, the starting PASI is way below 10.”
WHAT ARE YOUR THOUGHTS REGARDING THE 5-YEAR SAFETY DATA FOR TILDRAKIZUMAB?
“A lot of patients who have a long history of psoriasis are really afraid of the disease coming back,” said Dr Gerdes, and indeed, he continued, “we know from other psoriasis therapies that if we stop treatment, it will come back.” As such, he continued, “it is important for the patient to have a continuous, good, and efficacious therapy.” However, explained Dr Gerdes, “when they [patients] are happy their skin is clear then they continue their treatment, and after 1 or 2 years they might start questioning ‘do I still need this therapy? Is it safe if I take it over a long time?’”
Post hoc analysis of the reSURFACE 1 and 2 trials was carried out on the long-term safety data regarding severe infections, confirmed extended major adverse cardiovascular events, malignancies, and people ≥65 years of age (Table 1). They were reported as exposure-adjusted incidence rates, i.e., events per 100 patient-years of exposure.5-8
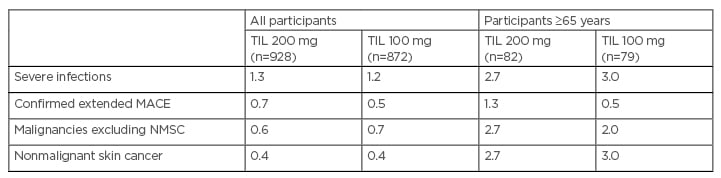
Table 1: Exposure-adjusted incidence rates for 5-year safety with tildrakizumab.5-8
Severe infections include any infection meeting the regulatory definition of an SAE and/or requiring intravenous antibiotics, irrespective of whether it was reported as an SAE. MACE include nonfatal myocardial infarction, nonfatal stroke, unstable angina, coronary revascularisation, resuscitated cardiac arrest, and cardiovascular deaths that were confirmed as ‘cardiovascular’ or ‘sudden’, and serious cardiovascular events.
MACE: major adverse cardiovascular events; NMSC: nonmelanoma skin cancer; SAE: serious adverse event;
TIL: tildrakizumab.
Both Dr Gerdes and Dr Maul agreed that the safety data were very convincing over the 5-year time period and reflected what they would expect from a general population, as well as being similar to data from other IL-23 inhibitors.20 “What’s most important,” highlighted Dr Gerdes, “is that we have the same safety signals in both dosages [of tildrakizumab], we don’t see a dose dependency. We also don’t see clusters [of adverse events of one type], we see a broad spectrum of different malignancies and infections.”5,7
Looking at the data for malignancies,7 Dr Gerdes explained how “we always look at malignancies like nonmelanoma skin cancer (NMSC) because we would expect this to be very prominent in psoriasis patients, especially if they had ultraviolet [light] therapies in the past or if they are older. It’s interesting that even the 5-year data is not showing a lot of NMSC.” These data are useful, he continued, “as we have conventional drugs that we would never use in patients who had a lot of ultraviolet [light] therapy in the past because we would be afraid they could develop NMSC; we didn’t see it at all here, as in other biologics.”
However, Dr Maul stressed: “We do need to take the real-world registry data into account and to have even longer time points of interpreting the safety.
Though 5 years is already very long, we always want 10 years, 15 years of data, from as many patients as possible.”
WHAT ARE YOUR OWN EXPERIENCES WITH TILDRAKIZUMAB IN TERMS OF EFFICACY, SAFETY, AND CONVENIENCE?
“Overall, I’m very happy with the IL-23 inhibitors,” reported Dr Maul. With this group of medications, she said, “there is a very good new opportunity for our psoriasis patients to be treated. What they enjoy a lot is that they don’t need to inject very often. With the IL-23 inhibitors, she explained, “they don’t really have the feeling that they suffer from the disease. The number of injections over the year is so limited.” Dr Gerdes confirmed this: “For patients, the therapy is very convenient. Here we inject when they are in the clinic, so they don’t have to do anything at home. Some of them start to forget they have psoriasis.”
Dr Gerdes has experience of more than 150 patients in his routine practice. He reported that “we have experienced a lot of very good results, the same or even better than we have seen in the clinical trials, with no new safety signals.” He also explained that, with his patients, “what we see is that the population is different from clinical trials, the PASI is way below 10 when they start treatments because a lot of patients are being switched from a previous therapy to a new one. If something doesn’t work really well, if they have a PASI of 8 or 9, we may stop a therapy and start a new one, we wouldn’t wait until they got to PASI 20.”
Dr Maul revealed that she currently prescribes tildrakizumab to around 21 patients, at the university hospital in Zurich, Switzerland, who are all included in the Swiss psoriasis registry (Swiss Dermatology Network for Targeted Therapies [SDNTT]). “I have a very positive experience with the treatment,” she reported. In fact, she continued, “the real-world data seems to be even better than we’ve seen in the clinical trial. From my experience, I’ve not had any severe safety problems, I’ve seen mild infections but nothing severe.”
CONCLUSION
In this discussion, Dr Gerdes and Dr Maul highlighted how IL-23 serves as a key regulatory cytokine for autoimmune inflammatory diseases such as plaque psoriasis because of its ability to stimulate survival and proliferation of Th17 cells. This upstream master cytokine has been targeted in the past few years with the advent of IL-23 inhibitors, including tildrakizumab, guselkumab, and risankizumab.
As a chronic condition, plaque psoriasis dictates long-term use of therapy that not only keeps symptoms at a level acceptable to the patient, preferably to a PASI score of less than 3, but that is also safe to use over an extended period of time. As Dr Maul and Dr Gerdes discussed, the absolute PASI score has become a preferable endpoint measure over the change in PASI scores, such as by 75% (PASI 75), that many clinical trials have used as it provides a real-world understanding of where a patient currently is in response to a therapy. They stressed that PASI scores should be just one measure of treatment response, with measures such as the DLQI also being of use in clinical practice to gain a more complete understanding of patient needs and goals.
Dr Gerdes and Dr Maul agreed that efficacy and safety data from the 5-year tildrakizumab reSURFACE 1 and 2 trials were very useful in understanding long-term aspects of such treatment. It was stressed how important it is to have such data as this gives clinicians confidence in prescribing a treatment to their patients with plaque psoriasis and gives the patient reassurance in using an IL-23 inhibitor for an extended period of time. Of note, for tildrakizumab, the data show that safety is not related to dose, with similar safety signals over 5 years for both 100 mg and 200 mg.
Both clinicians also highlighted the very good results observed with their patients receiving tildrakizumab, with the same or even better efficacy than has been observed in the clinical trials and with no new safety signals. However, they stressed that it is now important that real-world data is collected as every patient in daily practice is different.

![EMJ Dermatology 9 [Supplement 3] 2021 Feature Image](https://www.emjreviews.com/wp-content/uploads/2021/02/EMJ-Dermatology-9-Supplement-3-2021-Feature-Image-940x563.jpg)





