Meeting Summary
The oral session consisted of a talk on the 2-year efficacy and safety of guselkumab in the treatment of moderate-to-severe psoriasis from the Phase III VOYAGE 1 trial. This was followed by an oral session consisting of presentations regarding the clinical efficacy of tildrakizumab in patients with chronic plaque psoriasis over 2 years of treatment, presented from the two Phase III trials, reSURFACE 1 and 2. In the poster session, data were presented on the association between psoriasis and severity index measures, the distribution of improvement measures in psoriasis, and the efficacy of guselkumab in treatment-experienced patients, as well as its impact on the comorbidities of anxiety and depression.
Two-Year Efficacy and Safety of Guselkumab for the Treatment of Moderate-to-Severe Psoriasis: Phase III VOYAGE 1 Trial
Professor Andrew Blauvelt
Guselkumab is a selective interleukin (IL)-23 blocker that blocks the P19 subunit of IL-23. It was approved in the USA in July 2017 and by the European Medicines Agency (EMA) in November 2017 for the treatment of adult patients with moderate-to-severe plaque psoriasis. VOYAGE 11 was a Phase III, randomised, double-blind, placebo and active comparator-controlled trial. Patients were randomised to one of three treatment groups: guselkumab 100 mg subcutaneously at Week 0, 4, and every 8 weeks (Q8W) thereafter (n=329); placebo at Week 0, 4, 12, and then guselkumab 100 mg subcutaneously at Week 16, 20, and Q8W thereafter (n=174); or adalimumab 80 mg subcutaneously at Week 0, 40 mg at Week 1, and every 2 weeks thereafter up to Week 47, followed by guselkumab at Week 52 and Q8W thereafter (n=334). The data presented pertains to patients included in the VOYAGE 1 trial up to Week 100. Data analysis and the methodology used is of particular importance when determining long-term efficacy of agents. In the VOYAGE 1 trial,1 efficacy was prespecified to be assessed using non- responder imputation (NRI) through Week 48 and then application of treatment failure rules during Week 52–100. Treatment failure rules are not considered as conservative as NRI; however, they remain considerably more conservative than ‘as observed’ data.
All three treatment arms demonstrated very high Psoriasis Area Severity Index (PASI) 75 levels (approximately 95% of patients in all groups). Patients who were switched from placebo or adalimumab had the same outcomes as those patients who were treated with guselkumab for the entire 2-year period. Evaluating duration on treatment and durability analysis showed that 88% of patients who started on guselkumab remained on treatment at 2 years. Patients who switched to guselkumab from the placebo and adalimumab arms demonstrated a retention rate of 98%. Over 80% of all patients in all treatment arms achieved PASI 90 at 2 years. The number of patients who achieved PASI 100 was between 50% and 55%. The proportion of patients who achieved Investigator’s Global Assessment (IGA) 0 or 1 over the entire 2-year period was between approximately 82% and 85%, and correlated well with the PASI 90 data. There was little difference in outcomes between the treatment arms. IGA 0 correlated well with PASI 100.
Using less conservative but standard ‘as observed’ methodology, the proportions of patients achieving PASI 75, 90, and 100 were consistently higher than with NRI analysis. This was also seen in the IGA responses. Arguably, the NRI method of analysis is the most conservative analysis that can be used, but it is not consistent with real-world use and may underestimate the true efficacy of drugs, Prof Blauvelt said. He said that the closest data to true life data will in fact be somewhere between the ‘as observed’ and NRI numbers. The Dermatology Life Quality Index data demonstrated that the quality of life of patients on guselkumab continued to increase from Week 48–100. This was of particular interest in consideration of the efficacy data that showed that efficacy remained relatively consistent during this period of time. Adverse event (AE) rates in the second year were consistent with the first year of treatment. Serious AE rates were low and remained stable over time. There were no new safety concerns identified in the second year of treatment. Furthermore, the event rates per 100 patient-years remained consistent between the first and second years of treatment. In conclusion, efficacy of guselkumab was maintained over a 2-year period at high levels and it was well-tolerated with an acceptable safety profile.
Clinical Efficacy of Tildrakizumab, an Anti-IL-23P19 Monoclonal Antibody, in Patients with Chronic Plaque Psoriasis Over 2 Years of Treatment: Results From Long-Term Extensions to Two Phase III Clinical Studies (reSURFACE 1 and reSURFACE 2)
Professor Kim Papp
Prof Papp presented data from the reSURFACE 1 and 2 trials,2 demonstrating the maintenance and response in different strata (PASI 75, 90, and 100) in patients treated with tildrakizumab over 2 years. The base studies were three-part, double-blinded, randomised controlled trials. Patients were ≥18 years of age with moderate-to-severe chronic plaque psoriasis. Inclusion criteria comprised body surface involvement of ≥10%, a Physician’s Global Assessment (PGA) score of ≥3, and PASI ≥12. Patients were randomised to either tildrakizumab 100 or 200 mg or placebo in the dosing-finding reSURFACE 1 study (64 weeks) and reSURFACE 2 study (52 weeks), which compared tildrakizumab and etanercept.2
Patients who were identified as responders to tildrakizumab were enrolled in the extension studies. Patients needed to have achieved PASI ≥50 and have received an active dose within 12 weeks of the end of the study in the reSURFACE 1 extension trial. Open-label treatment continued at 100 or 200 mg every 12 weeks. In the reSURFACE 2 trial, responders to etanercept were not switched to tildrakizumab and were not enrolled in the extension study.
The primary efficacy population in the extension studies consisted of the full analysis set (patients with ≥1 dose of extension treatment based on assigned treatment). The primary safety population consisted of all subjects as treated (patients with ≥1 dose of extension treatment based on treatment received). The efficacy objective for the extension trials was to evaluate the maintenance of efficacy response levels, prespecified based on observed data. The safety objectives were to evaluate pre-specified adverse events of interest, and to determine yearly and cumulative incidence rates. The data presented were the interim 2-year data of the planned 5-year total extension trials.
The baseline characteristics and demographics were similar across both studies and between the treatment arms. Prof Papp suggested that the similarity in baseline characteristics would predict similar levels of maintenance and response between studies. Patients in reSURFACE 1, up to 2 years, demonstrated a maintained PASI 75 response at Week 48 in the extension period at both doses, compared with the end of the base study. Due to the low drop-out rate, the ‘as observed’ method was sufficiently robust to suggest the data is a good representation of the expected maintenance response, he said. Both trials demonstrated a gradual but minimal level of decline in PASI 75. The absolute efficacy values after 2 years of treatment were slightly different between reSURFACE 1 and 2. The maintenance response remained acceptable during this period. In reSURFACE 2, approximately one-third of patients maintained PASI 100 and approximately two-thirds of patients maintained PASI 90. The overall efficacy data from the reSURFACE 1 and 2 extension trials of PASI 75, 90, and 100 demonstrated a convincing maintained response over 2 years, he said.
The safety data from both extension trials showed an acceptable safety ‘profile’ at both doses. The 2-year cumulative number of patients with AE of interest remained low. Severe infections, which are of particular interest in treatments that modify, modulate, or suppress the immune system, also remained low at both doses. Prof Papp suggested the rate of severe infections appeared to be independent of dose, based on observations of the 2-year interim data presented.
Overall, the reSURFACE 1 and 2 extension trials 2-year interim efficacy data demonstrated convincing maintenance of efficacy in the treatment of chronic plaque psoriasis. Furthermore, the cumulative 2-year safety observations indicated that both the 100 and 200 mg doses were well-tolerated with low rates of AE of interest.
Question and Answer Session
Q: Are there any particular phenotypes of psoriasis that did not respond to treatment, because there still appear to be a few patients who did not respond?
A: Prof Papp replied: “The whole discussion surrounding phenotype is complicated. The complexity of the disease shows that phenotype drift can occur even within patients. The focus should be on the instruments used. The PASI score, for example, is incredibly useful yet deficient in its ability to capture the lower ranges of responses. This can translate into the exclusion of certain phenotypes, e.g., widespread psoriasis that is not very inflamed, thin, or scaly. An instrument that could identify the different phenotypes would be very useful; however, currently a tool that is sensitive enough does not exist.”
Poster Presentation P1726: Association Between Psoriasis Area Severity Index and Physician’s Global Assessment Responses in Moderate-to-Severe Chronic Plaque Psoriasis Studies of Tildrakizumab
Professor Doctor Kristian Reich
Tildrakizumab is an anti-IL23p19 monoclonal antibody under development for the treatment of moderate-to-severe chronic plaque psoriasis. It has been shown to significantly improve PASI 75, 90, and 100, and PGA response rates when compared with placebo.2,3 PASI and PGA are the most commonly used measures of psoriasis severity but an association study between the two measures has not been conducted for treatment with tildrakizumab. The objective of the study presented was to determine if there was an association between the two measures using tildrakizumab data from two Phase III trials, reSURFACE 1 and reSURFACE 2.
The patients in the reSURFACE 1 and 2 trials were adults with moderate-to-severe plaque psoriasis and with ≥10% body surface area, a PGA score of ≥3, and PASI ≥12. The primary endpoints of both studies were the proportion of patients achieving PASI ≥75 and the proportion of patients achieving a PGA score of ‘clear’ (0) or ‘minimal’ (1) with a >2 grade reduction from baseline at Week 12. Secondary endpoints included the proportion of patients achieving PASI 90 and 100 at Week 12, and PASI 75 and a PGA score of 0 or 1 with a >2 grade reduction from baseline at Week 12 and 28 versus etanercept (reSURFACE 2).
Response was analysed using the Cochran-Mantel-Haenszel test stratified by weight and prior use of biologics for psoriasis. NRI was prespecified at Week 12. At Week 28, NRI was prespecified for PASI 75 and PGA response in reSURFACE 2. Analysis of observed data was prespecified for PASI 75, 90, 100, and PGA response in reSURFACE 1, and PASI 90 and 100 in reSURFACE 2. Data were pooled from patients in all treatment arms with both PASI and PGA data at baseline at Week 12 and 28.
A total of 772 and 1,090 patients were included in reSURFACE 1 and 2, respectively. There was a statistically significant association between PASI 75, 90, and 100 responses, and PGA 0 or 1 responses at Week 12 and 28 (p<0.001 for all). Furthermore, the association between PASI 100 and PGA response was higher compared with PASI 75 or 90 and PGA response (Table 1). In summation, there was a significant association between PASI 75, 90, or 100 responses, and achievement of PGA 0 or 1 in tildrakizumab-treated patients at Week 12 and 28.
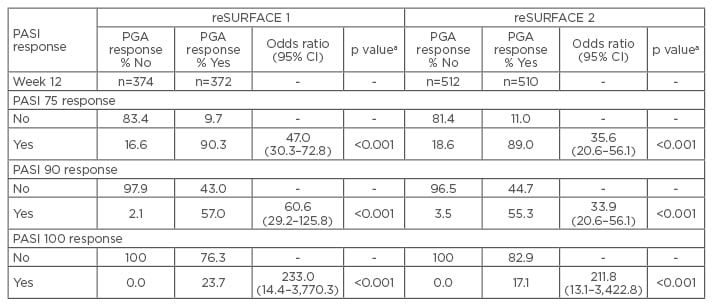
Table 1: Association between the proportion of patients with Psoriasis Area Severity Index and Physician’s Global Assessment response at Week 12.
ap values not adjusted for multiplicity.
CI: confidence interval; PASI: Psoriasis Area Severity Index; PGA: Physician’s Global Assessment.
Poster Presentation P1812: Distribution of Improvements in Psoriasis Area Severity Index from the Phase II Trial of Risankizumab in Moderate-to-Severe Plaque Psoriasis
Professor Bruce Strober
Risankizumab is a humanised immunoglobulin G1 monoclonal antibody, which is an IL-23 inhibitor. A phase II trial demonstrated its superiority over ustekinumab in patients with moderate-to-severe plaque psoriasis.4 PASI 90 is often used as a primary endpoint in clinical trials as a measure of treatment efficacy. It is suggested that additional visualisation of the cumulative distribution of responses can help assess the consistency of PASI at the population level. The objective of this study was to determine the distribution of PASI responses in patients treated with risankizumab compared with ustekinumab in a Phase II trial.
The Phase II trial consisted of patients with moderate-to-severe plaque psoriasis (n=166) randomised to receive subcutaneous injections of risankizumab (either 18 mg single dose, 90 mg, or 180 mg, at Week 0, 4, and 16) or ustekinumab (45 or 90 mg based on body weight at Week 0, 4, and 16). The proportion of patients who achieved PASI 75, 90, or 100 were assessed at Week 12 and 16 in an intent-to-treat population. Those patients with missing assessments were defined as non-responders. Cumulative probability plots were used to determine the distribution of changes in PASI from baseline across the treatment groups.
At Week 12, 30.2%, 73.2%, and 78.6% of patients achieved PASI 90 for 18, 90, and 100 mg risankizumab, respectively, compared with 40.0% in ustekinumab-treated patients. Patients who were treated with 90 or 180 mg risankizumab achieved higher response rates across all levels of PASI compared with patients treated with either 18 mg risankizumab or ustekinumab. The cumulative probability plot demonstrated that patients treated with 90 or 180 mg risankizumab had a higher probability of increased improvements in PASI scores from baseline compared with patients treated with 18 mg risankizumab or ustekinumab, as seen in Figure 1.
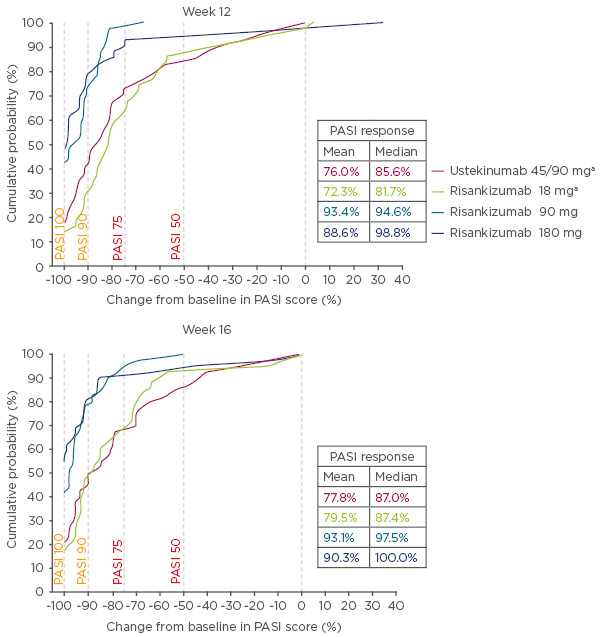
Figure 1: Cumulative probability of percentage change from baseline in Psoriasis Area Severity Index scores at Week 12 and 16.
aOne patient each with missing response in risankizumab 18 mg and ustekinumab 45/90 mg groups, and were imputed as having no (0%) improvement.
PASI: Psoriasis Area Severity Index.
Poster Presentation P1813: Guselkumab Demonstrates Greater Reductions in Anxiety and Depression Symptoms Than Adalimumab in Psoriasis Patients
Professor Kenneth Gordon
Psoriasis is associated with anxiety and depression; improvements in psoriasis lesions have been shown to decrease these comorbidities.5,6 Guselkumab has demonstrated efficacy and safety in the treatment of patients with moderate-to-severe plaque psoriasis.1,7 The study presented here aimed to evaluate whether guselkumab improved the symptoms of anxiety and depression using the Hospital Anxiety and Depression Scale (HADS), compared with placebo and adalimumab-treated patients from the VOYAGE 2 trial. The study further aimed to evaluate any correlations between anxiety or depression scores and efficacy measures.
VOYAGE 2 was a Phase III, double randomised, double-blind, study. Patients were randomised to one of three treatment arms: guselkumab 100 mg subcutaneously at Week 0, 4, 12, and 20 (n=496); placebo at Week 0, 4, 12, followed by guselkumab 100 mg subcutaneously at Week 16 and 20 (n=248); or adalimumab 80 mg subcutaneously at Week 0, 40 mg at Week 1, and then every 2 weeks to Week 23 (n=248). Efficacy was assessed through Week 24. The anxiety and depression HADS subscales (HADS-A and HADS-D, respectively), consisted of seven questions scored 0–3. Total scores ranged from 0–21 with higher scores reflecting increased severity. Scores ≥8 are the instrument definition of anxiety or depression. PASI was used to assess psoriasis severity. Patients with HADS scores ≥8 were evaluated for correlations between change in anxiety or depression scores with percentage improvement in PASI scores.
A total of 992 patients were randomised at baseline. The mean HADS-A and HADS-D scores were 6.8 and 5.3, respectively; a total of 38.6% and 27.7% of patients had HADS-A and HADS-D scores ≥8, respectively. Baseline characteristics were similar between treatment arms. The findings showed that patients treated with guselkumab demonstrated significantly greater improvements in both HADS scores at Week 8 and 16, and in HADS-A scores in Week 24 (p<0.001 for all). Significantly greater proportions of patients in the guselkumab arm with baseline HADS scores of ≥8 reported HADS scores <8 compared with placebo at Week 16 (p<0.001 for both HADS scores) and compared with adalimumab at Week 24 (p=0.028 for HADS-A and p=0.079 for HADS-D). Overall improvements in PASI scores showed significant correlations with change from baseline in anxiety (r=0.27) and depression (r=0.25) at Week 24 (p<0.001 for all).
The findings from this study showed that guselkumab demonstrated greater improvements in the comorbidities, anxiety and depression, in patients with moderate-to-severe plaque psoriasis when compared with placebo and adalimumab. These improvements were correlated with improvements in psoriasis.
Poster Presentation P1830: Efficacy of Guselkumab in Previously Treated Patients with Moderate-to-Severe Plaque Psoriasis: An Analysis from VOYAGE 1 and VOYAGE 2
Professor Kenneth Gordon
Guselkumab therapy has demonstrated superior clinical responses (p<0.001) compared with placebo and adalimumab in the Phase III clinical trials VOYAGE 1 and 2.1,7 The study described here presented data from these trials evaluating the efficacy of guselkumab compared with placebo and adalimumab in psoriasis treatment-naïve and experienced patients. A total of 1,829 patients were randomised in VOYAGE 1 (n=837) and 2 (n=992) to the following treatment groups: guselkumab 100 mg at Week 0, 4, 12, and 20 (n=825); placebo at Week 0, 4, 12, followed by guselkumab 100 mg at Week 16 and 20 (n=422); or adalimumab 80 mg at Week 0, 40 mg at Week 1, and 40 mg every 2 weeks thereafter through Week 23 (n=582). Data from both trials were pooled for analysis. Prior psoriasis therapies included non-biologic systemics, biologics, non-biologic systemics or biologics, anti-tumour necrosis factor, biologics etanercept and infliximab, and IL-12/23 inhibitors. Efficacy assessments performed at Week 16 and 24 included IGA scores of 0 or 1 and PASI 90.
Baseline characteristics were similar between treatment arms. The majority of patients had psoriasis for >17 years and PASI and IGA scores were consistent with a population with moderate-to-severe psoriasis. Over 50% of patients had used phototherapy and non-biologic systemics, approximately 20% had used biologic therapies, and approximately 10% had used anti-tumour necrosis factor biologics and IL-12 or IL-23 inhibitors. Regardless of previous psoriasis treatment experience, significantly greater proportions of patients in the guselkumab treatment arm achieved IGA scores 0 or 1 and PASI 90 compared with placebo (p<0.001) and adalimumab (p<0.001) at Week 16, as seen in Figure 2. Both IGA and PASI 90 outcomes were generally similar between all guselkumab-treated patients, regardless of previous treatment experience, at Week 16 and 24.
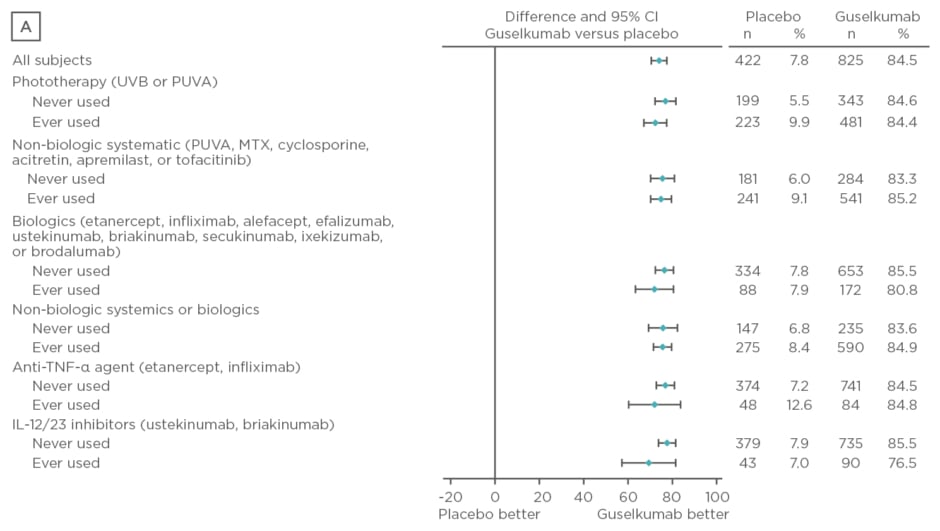
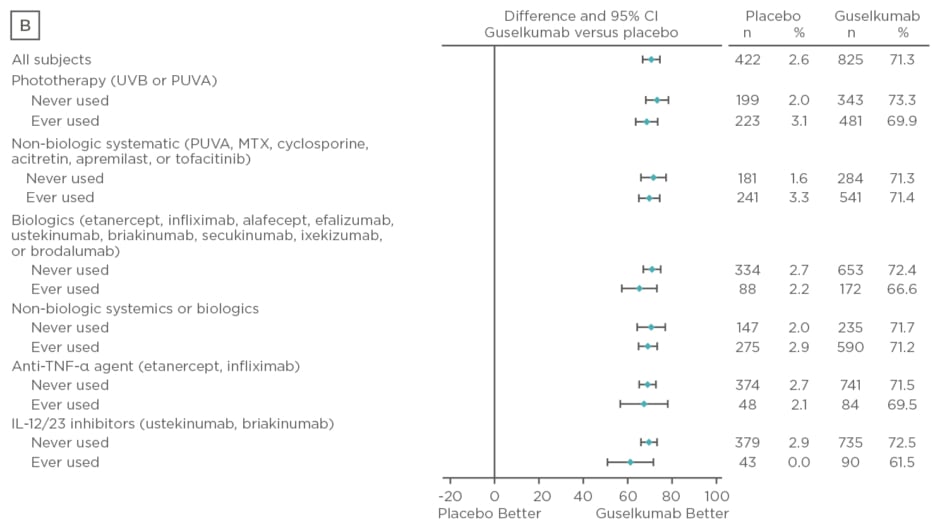
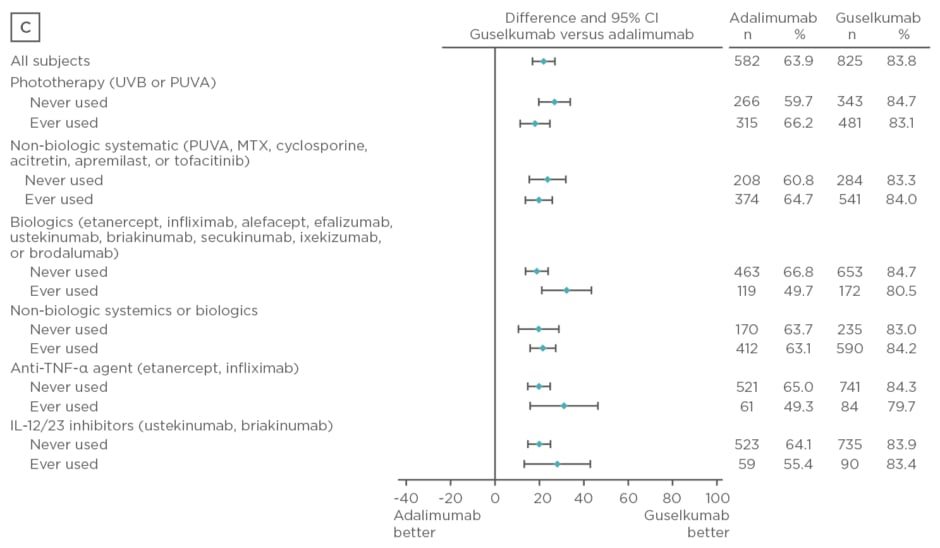
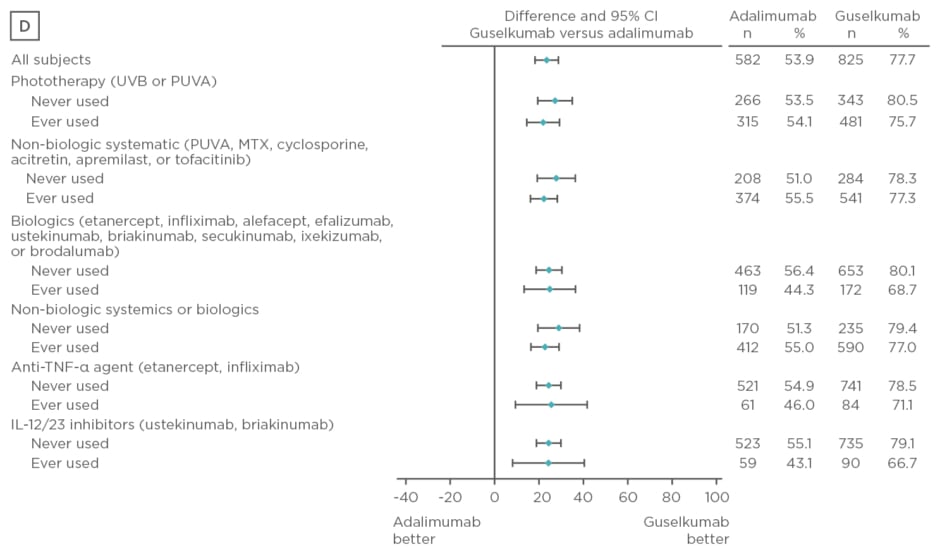
Figure 2: Proportion difference and 95% confidence intervals for comparing proportion of patients achieving Investigator’s Global Assessment and Psoriasis Area Severity Index scores.
A) IGA score 0/1 at Week 16 by treatment experience (placebo versus guselkumab); B) PASI 90 at Week 16 by treatment experience (placebo versus guselkumab); C) IGA score 0/1 at Week 24 by treatment experience (adalimumab versus guselkumab); D) PASI 90 at Week 24 by treatment experience (adalimumab versus guselkumab).
CI: confidence interval; IL: interleukin; IGA: Investigator’s Global Assessment; MTX: methotrexate; PASI: Psoriasis Area Severity Index; PUVA: psoralen and ultraviolet A; TNF-α: tumour necrosis factor-alpha; UVB: ultraviolet B.
Overall, treatment with guselkumab was well tolerated and the safety results were comparable between treatment-naïve and experienced patients. Guselkumab demonstrated superiority to placebo at Week 16 and adalimumab at Week 24 regardless of previous therapy experience.