Meeting Summary
This presentation by Dr Weisenseel considered the role of fumaric acid esters (FAE) in plaque psoriasis. FAE, first developed in 1959 and approved in 1994, are available in two forms: dimethyl fumarate (DMF) alone (e.g. Skilarence®; Almirall Ltd., Uxbridge, UK) or DMF together with calcium, zinc, and magnesium salts of monoethyl fumarate (Fumaderm®). Up-titration is recommended for FAE/DMF dosing. The BRIDGE study demonstrated that Skilarence has comparable efficacy, safety, and tolerability to Fumaderm, while the retrospective FUTURE study demonstrated that the efficacy of FAE increase over time. Additionally, in separate studies combining FAE with ultraviolet therapy, FAE were shown to achieve a faster clinical response and required a lower mean maximum daily dose in the up-titration period. FAE side effects, such as flushing and gastrointestinal effects, can usually be handled by individualising patient doses, involving both up and down-titration. Dr Weisenseel explained how patients are required to have their lymphocyte levels monitored every 3 months, with lymphocyte counts <700 cells/µL in two consecutive tests considered the criterion for stopping DMF therapy due to the increased risk of progressive multifocal leukoencephalopathy (PML). There is no evidence of drug-drug interactions with FAE, although retinoids, cyclosporin, immune suppressants, and cytostatics should be avoided.
Dimethyl Fumarate in Psoriasis Therapy
Doctor Peter Weisenseel
According to the German psoriasis registry PsoBest, FAE are the most commonly used systemic psoriasis treatment in Germany.1 Fumarates were first developed in 1959 by Walter Schweckendiek, a German chemist who experimented with different FAE mixtures to treat his own psoriasis.2 Although individual clinicians used FAE for psoriasis in the 1960s,3 it was not until 1989 that data from the first controlled clinical study were published.4 Approval of the first FAE compound, Fumaderm, took place in Germany 1994,5,6 followed by the fumarate Tecfidera® (Biogen, Cambridge, Massachusetts, USA), for multiple sclerosis (MS) in the USA and the European Union (EU) in 2014.7 Most recently, the EU approved the DMF Skilarence for the treatment of psoriasis in 2017.8 Fumaderm contains DMF together with calcium, zinc, and magnesium salts of monoethyl fumarate, while Skilarence only contains DMF.6,8 Although the mechanism of action is yet to be clearly elucidated, DMF is thought to bring about a shift in dendritic cell maturation from type 1 to type 2 cells and promotes the maturation of immature T cells into Th2 over Th1.9
Up-titration is recommended for FAE/DMF dosing.10 In the first week of Skilarence therapy, a 30 mg dose is administered once daily (a single tablet every evening). During Week 2, the 30 mg Skilarence dose is administered twice daily (one tablet in the morning and one in the evening). During Week 3, a 30 mg Skilarence dose is administered three times daily (one tablet in the morning, one at midday, and one in the evening). From Week 4, treatment is switched to a single 120 mg dose of Skilarence administered in the evening, and the dose is then increased by one 120 mg Skilarence tablet per week, administered at the three different timepoints for the subsequent 5 weeks, up to a maximum daily dose of 720 mg (two 120 mg Skilarence tablets administered three times a day).8 However, a daily dose of 360–480 mg is usually sufficient, with the exception of overweight patients. Clinicians and patients need to appreciate that FAE take time to work, with the onset of efficacy being ‘a marathon more than a sprint’.
In Germany, when a stable clinical response has been achieved, titration is slowed to the minimum efficacious dose recommended, with patients taking one fewer tablet per day every 1–3 months until there is a slight relapse (evidenced by the return of spots), when the dose is increased to that prior to the relapse.11 Psoriasis disease activity can vary according to seasons, infections, or new hypertensives, making it helpful for patients to be able to up or down-titrate treatment.
The BRIDGE study demonstrated that Skilarence has comparable efficacy to Fumaderm.12 During the study, 704 patients with moderate-to-severe psoriasis were randomised 2:2:1 to receive Skilarence (n=267), Fumaderm (n=283), or a placebo (n=137). Results at Week 16 showed 37.5% of patients treated with Skilarence achieved Psoriasis Area Severity Index (PASI) 75, compared with 15.3% receiving placebo (superiority for Skilarence versus placebo p<0.001), and 40.3% receiving Fumaderm (non-inferiority for Skilarence versus Fumaderm p<0.001). Furthermore, safety and tolerability data were comparable for Skilarence and Fumaderm. The most common treatment emergent adverse events were diarrhoea (Skilarence: 38.7%; Fumaderm: 39.9%; placebo: 16.8%) and upper abdominal pain (Skilarence: 20.1%; Fumaderm: 22.5%; placebo: 8%). An important finding at Week 16 was that Skilarence and Fumaderm continued to show an upward trend, indicating maximum efficacy had yet to be reached, and, as such, it would have been advisable to perform the final assessment at Week 24 (Figure 1).
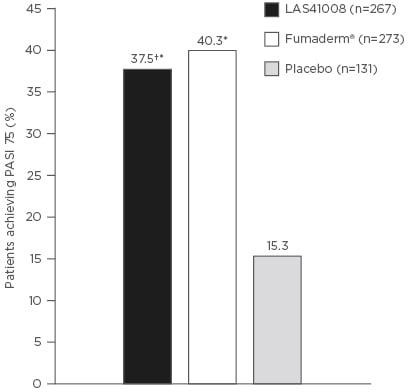
Figure 1: Percentage of patients achieving ≥75% improvement in Psoriasis Area Severity Index (PASI 75) at Week 16 (full analysis set).
*p<0.001 versus placebo; †p<0.001 non-inferiority versus Fumaderm®.
Adapted with permission from Mrowietz et al.12
During psoriasis treatment, not only is it important to achieve good efficacy at the start but also to achieve good long-term performance, avoiding the need to switch treatments. An expert consensus identified that the time taken to achieve optimal clinical response varies, with the panel suggesting efficacy assessments for fast-acting drugs (infliximab, adalimumab, and ustekinumab) at Week 16, while assessments for slow-acting drugs (methotrexate, etanercept, and fumarates) take place at Week 24.13 In the German S3-guidelines (in which factors including safety/tolerability, practicability, and costs are taken into consideration) fumarates perform well and in certain aspects are similar to biologics.10
In 2015, the EU guidelines included recommending FAE for long-term psoriasis treatment. The FUTURE study,14 a retrospective analysis of 984 psoriasis patients who had undergone ≥2 years’ continuous FAE treatment, demonstrated how treatment benefits increase with time. After 6, 24, and 36 months of therapy, 67%, 78%, and 82% of patients were documented as ‘markedly improved or clear’, respectively. An analysis of FUTURE data exploring mean PASI improvement according to whether patients had severe psoriasis (PASI >20), moderate-to-severe psoriasis (PASI 10–20), and mild psoriasis (PASI <10) found all grades showed improvement after 6 and 12 months.14
One way to achieve a faster treatment response is the combination of FAE with ultraviolet therapy. Psoriasis patients in the FAST study15 treated with FAE and ultraviolet light showed faster clinical responses (improvement of mean PASI) than patients in the FUTURE study,14 who were treated with FAE monotherapy. After 12 months, there was no difference in efficacy between the groups.
Two studies have demonstrated good long-term drug survival for FAE.16,17 A German study showed FAE have the highest drug survival of conventional systemics,16 while an Irish retrospective study showed that FAE have a 4-year drug survival rate of 60%, comparing favourably with reported 4-year survival rates of 40% for etanercept and adalimumab and 70% for infliximab.17 Survival benefits for FAE contrast with strong biologics, which, although initially powerful, show secondary lack of response after 1 or 2 years.
Regarding safety, the PsoBest registry identified no significant differences for non-severe infection, severe infection, serious infection, non-melanoma skin cancer, and malignancies between FAE and other treatments, including adalimumab, etanercept, infliximab, anti-TNF, ustekinumab, biologics, methotrexate, cyclosporine, and systemics.18
Side Effects of Fumaric Acid Esters
Clinicians talking to patients starting FAE could use the analogy of mountains and lakes, cautioning that they will have to pass over ‘a hill or mountain of side effects’ before encountering the ‘lake of efficacy’. The most common FAE side effects are flushing and gastrointestinal problems, although it is impossible to predict the extent of these side effects. Flushing is the spontaneous and transient reddening of the face and/or neck, occurring 1–5 hours after FAE intake. The effect, believed to involve prostaglandin-mediated vasodilatation, occurs less often during long- term therapy.19 Recommendations for avoiding flushing include taking the highest FAE daily doses in the evening and using prophylactic prostaglandin inhibitors (e.g., acetyl salicylic acid 325–500 mg). Gastrointestinal side effects (e.g., diarrhoea, nausea, and bloating) usually start from the 120–240 mg DMF per day dose range but fade with long-term therapy.19 Recommendations to reduce gastrointestinal effects include decreasing the dose to the last well-tolerated dose, taking the highest daily dose in the evening, and consuming the drug with dairy products. Some patients benefit from antihistamines or proton-pump inhibitors.
Patients starting Skilarence are required to have lymphocyte and leukocyte counts ≥1,000 cells/µL and ≥3,000 cells/µL, respectively, and to be monitored every 3 months. If lymphocyte levels fall below 1,000 cells/µL but remain >700 cells/µL, blood tests should be undertaken every month. If a patient’s lymphocytes count is <700 cells/µL on two consecutive tests, Skilarence should be stopped and the patient prescribed another therapy.8 The motive for monitoring is to avoid sustained low lymphocyte counts, which could put the patient at risk of developing a very rare viral infection of the central nervous system called PML. PML, caused by reactivation of the John Cunningham virus, has been reported after administration of immunosuppressive drugs and also in patients with very low lymphocyte counts (<500 cells/µL). Although PML has been most widely reported with natalizumab in MS (>750 cases), 19 PML cases have been associated with FAE since 1994, including Fumaderm and Tecfidera.20,21
One beneficial aspect of FAE treatment is metabolism by unspecific esterases; as a result, there are no direct drug–drug interactions.22 However, due to FAE influence on the immune system and kidney function, retinoids, cyclosporin, immune suppressants, and cytostatics should be avoided during FAE treatment.6
Conclusion
In Dr Weisenseel’s personal experience, patients not considered optimal for FAE include those who have a past of many prior systemic therapies, acute/severe flares, or psoriatic arthritis, as well as those who expect a fast onset of action. Patients considered optimal include those with chronic/stable psoriasis, a history of good treatment compliance, and those looking for good long-term psoriasis control.
Question and Answer Session
Answering a question about the profile of patients prescribed FAE, Dr Weisenseel said they usually have chronic, stable disease with no acute flares or arthritis. Biologics are preferable for severe patients wanting a fast onset of action, but some patients are deterred by perceptions of methotrexate as a former ‘anti-cancer drug’. The second question related to the management of patients experiencing both psoriasis and MS, with Dr Weisenseel explaining that he had tried treatment with FAE previously but switched to a biologic after the patient had a MS relapse. While patients have been treated with combinations of conventional oral therapies with biologics (such as etanercept and methotrexate), this combination can result in blood cell count and kidney function complications.
Considering patients with prolonged lymphopenia on fumarates, Dr Weisenseel said that it rarely takes more than several months after coming off the drug before blood cell counts recover. There is no evidence, he added, that lymphopenia interferes with FAE efficacy. Regarding combination therapy, Dr Weisenseel stated that it is reasonable for patients needing a fast onset of action to combine FAE with phototherapy, and that there is no risk of phototoxicity. However, although there have been case reports of this combination treatment, he advised against combining FAE with other systemic therapies.







