Presenters: Bruce E. Strober,1,2 Daniel Aletaha,3 Jo L.W. Lambert4
1. Department of Dermatology, Yale University, New Haven, Connecticut, USA
2. Central Connecticut Dermatology Research, Cromwell, USA
3. Division of Rheumatology, Department of Medicine 3, Medical University of Vienna, Austria
4. Department of Dermatology, Ghent University Hospital, Ghent, Belgium
Disclosure: Strober has received honoraria from AbbVie, Almirall, Amgen, Arcutis, Arena Pharmaceuticals, Aristea Therapeutics, Asana BioSciences, Boehringer Ingelheim, Eli Lilly and Company, Immunic Therapeutics, Bristol Myers Squibb, Connect Biopharma, Dermavant Sciences, Equillium, GlaxoSmithKline, Janssen, LEO Pharma, Maruho Co., Ltd., Meiji Seika, Mindera Health, Novartis, Pfizer, UCB, Sun Pharma, Ortho Dermatologics, Regeneron, and Sanofi-Genzyme; research funding from AbbVie, Cara Therapeutics, CorEvitas Psoriasis Registry (formerly Corrona Psoriasis Registry), Dermavant Sciences, Dermira, and Novartis; and is the co-scientific director of the CorEvitas Psoriasis Registry (formerly Corrona Psoriasis Registry). Aletaha has received research funding from AbbVie, Novartis, and Roche; is a consultant for AbbVie, Amgen, Celgene, Eli Lilly and Company, Medac, Merck, Novartis, Pfizer, Roche, Sandoz, and Sanofi-Genzyme; and has received speaker fees from AbbVie, Celgene, Eli Lilly and Company, Merck, Novartis, Pfizer, Sanofi-Genzyme, and UCB. Lambert has received research funding from AbbVie, Amgen, Janssen, LEO Pharma, Novartis, and UCB; and conference fees from AbbVie, Bristol Myers Squibb, Janssen, and UCB.
Acknowledgements: Medical writing assistance was provided by Robert Johnson of Excerpta Medica, Amsterdam, The Netherlands.
Disclaimer: The opinions expressed in this article belong solely to the named authors.
Support: The symposium and publication of this article were funded by Janssen. The views and opinions expressed are those of the speakers and not necessarily of Janssen.
Citation: EMJ Dermatol. 2022;10[Suppl 1]:2-10.
Meeting Summary
This Janssen-sponsored virtual satellite symposium entitled ‘Diving deep: Harnessing the Potential of the IL-23 Pathway’ took place at the virtual 30th European Academy of Dermatology and Venereology (EADV) Congress 2021.
The symposium focused on IL-23 as a therapeutic target for the treatment of psoriasis and psoriatic arthritis (PsA) due to the pivotal role played by IL-23 in the IL-23/Th17 signalling axis in the development of disease pathologies.
Bruce E. Strober discussed some of the latest data to emerge regarding long-term disease remission following the inhibition of IL-23 in patients with psoriasis. Most notably, the high rates of Psoriasis Area Severity Index (PASI) responses that are maintained through long-term follow-up offer patients the possibility of sustained skin clearance with low-frequency dosing.
Similar developments were reported for IL-23 inhibition in patients with PsA, despite the greater heterogeneity of this disease. Daniel Aletaha highlighted the pattern of increasing American College of Rheumatology (ACR) response rates through 2 years of treatment with guselkumab, with benefits observed across clinical and patient-reported outcomes regardless of prior treatment experience.
Maximising the value offered by such treatment options requires a considered approach and Jo L.W. Lambert discussed how the value-based healthcare model with integrated practice units can be adopted to optimise outcomes. The importance of patient involvement within a support network was emphasised, alongside the need for greater reporting and sharing of data to facilitate improved outcomes across patient populations.
Perspectives from Dermatology: The Potential of IL-23 Inhibitors for Long-Term Disease Remission
Bruce E. Strober
Strober began by summarising his opinion on the key characteristics of anti-IL-23 therapies that support their efficacy for inducing long-term disease remission in patients with psoriasis. Namely, the possibility of infrequent dosing (i.e., every 8–12 weeks), including tolerance towards variable dosing, a long-term response rate that has demonstrated superiority over IL-17 and TNF inhibition, sustained efficacy following treatment withdrawal, and a mechanism of action that leads to an altered immune phenotype and the deep suppression of the inflammatory cytokines that drive disease pathogenesis. He went on to discuss evidence from which these observations were derived.
The long-term impact of IL-23 inhibition via infrequent dosing has been clearly demonstrated in VOYAGE 2, a Phase III trial in which patients with moderate-to-severe plaque psoriasis who were randomised to guselkumab (100 mg at Weeks 0 and 4, and then every 8 weeks [q8w]) at baseline, or who crossed over to guselkumab following initial randomisation to placebo (Week 16), or adalimumab (Week 28), achieved high rates of PASI 90 through 5 years (82.0% for the guselkumab and placebo to guselkumab group versus 79.1% for the adalimumab to guselkumab group; Figure 1). Also of note are the high rates of study retention, with only 15% drop off from Week 100–252 of the study. Safety profiles were consistent between groups with low rates of discontinuation due to adverse events (AEs).1
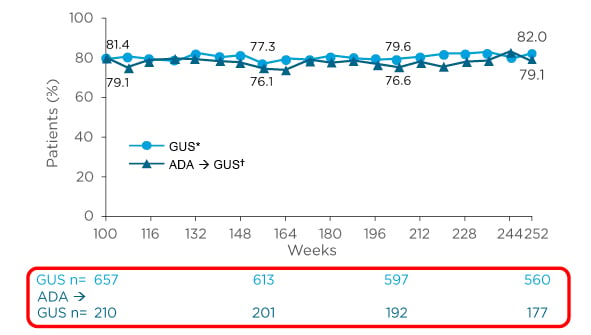
Figure 1: Maintenance of PASI 90 response in patients with psoriasis with up to 5 years of continuous guselkumab treatment.
*Includes patients randomised to guselkumab or placebo at baseline who crossed over to receive guselkumab at Week 16.
†Includes patients randomised to adalimumab at baseline who crossed over to receive guselkumab at or after Week 28.
Adapted from Reich K et al.1
ADA: adalimumab; GUS: guselkumab; PASI 90: ≥90% improvement in Psoriasis Area Severity Index.
A similar pattern of increasing efficacy over time with guselkumab has been reported in biologic-naïve patients with PsA, with ACR20, 50, and 70 responses of 74%, 55%, and 36%, respectively, observed through Week 100 following treatment with guselkumab q8w in the Phase III trial DISCOVER-2.2
The superiority of inhibiting IL-23 over other targets has been clearly demonstrated in head-to-head trials of patients with psoriasis. VOYAGE 1 demonstrated superiority of guselkumab over adalimumab (80 mg at Week 0, followed by 40 mg at Week 1, and then 40 mg every 2 weeks through Week 47) in PASI 75 and 90 response rates at Week 16. Numerically higher values with guselkumab versus adalimumab were maintained through Week 48 for PASI 75 (87.8% versus 62.6%), PASI 90 (76.3% versus 47.9%), and PASI 100 (47.4% versus 23.4%) response rates (all p<0.001 versus adalimumab at Week 48).3 In addition, the Phase III trial ECLIPSE demonstrated the superiority of guselkumab (100 mg at Weeks 0, 4, and then q8w) over secukinumab (300 mg at Weeks 0, 1, 2, 3, 4, and then every 4 weeks [q4w]) in PASI 90 response rates at Week 48 (84% versus 70%; p<0.0001). It is noteworthy that although secukinumab demonstrated a slightly more rapid response (PASI 90 response rates of 69% versus 76% at Week 12), response rates in this group appeared to begin to drop after Week 20, while those of the guselkumab group continued to increase and were maintained through Week 48.4 These data reflect those of other IL-23 inhibitors versus secukinumab, whereby differences in PASI90 response rates were greater at Week 52 than Week 16 in favour of mirikizumab versus secukinumab (OASIS-2)5 and risankizumab versus secukinumab (IMMerge),6 with IL-23 inhibitors consistently achieving PASI 90 response rates of >80% at 1 year.4-6
The enhanced efficacy and safety of IL-23 inhibitors was also evidenced by a retrospective analysis of 16 cohorts that assessed drug survival across different drug classes. While drug survival was high for all classes at 6 months, IL-23 inhibitors demonstrated a greater cumulative rate of drug survival than other biologics at 18 months: risankizumab 96.4% and guselkumab 91.1%; versus brodalumab 86.3%; ustekinumab 86.1%; ixekizumab 82.0%; and secukinumab 79.9%.7
Patients may need to interrupt therapy for multiple reasons,8 with IL-23 inhibitors shown to have a long duration of remission after drug withdrawal.9 This is evidenced by median time from last dose of drug to loss of PASI 90 of 28–32 weeks for tildrakizumab, 23 weeks for guselkumab, and 36 weeks for risankizumab (estimate based on time to 52.4% PASI 90 responders).10-12 Such activity may be explained by the deep and durable impact of the mechanisms by which IL-23 inhibition act.13
Although multiple immune axes are involved in the pathogenesis of psoriasis, it is the IL-23/Th17 axis that drives the disease phenotype, and this begins to explain the mechanisms of action that drive the characteristics of IL-23 inhibition.14-16 IL-23 acts upstream of IL-17, driving alternative outcomes of Th17 differentiated cells, in particular cell death, altered Th17 phenotype, and conversion to regulatory T cells, each of which has an inhibitory effect on psoriasis pathology.4,17
The differential downstream cellular and molecular impacts of IL-23 versus IL-17 inhibition were examined in mechanistic sub-studies of the ECLIPSE trial. In these analyses, both guselkumab and secukinumab led to significant decreases in the gene expression of pro-inflammatory cytokines IL-17A and IL-22 from baseline to Week 24 in lesional skin. However, guselkumab led to more rapid and significantly greater reductions in serum IL-17F and IL-22 versus secukinumab at Week 24 and Week 48, with levels of IL-17F in particular approaching those of healthy controls.17 The ‘molecular scar’ of psoriatic skin describes a transcriptome of 3,575 differentially expressed transcripts between lesional and non-lesional skin. Guselkumab and secukinumab were both associated with a normalisation of the transcriptome from lesional to non-lesional skin. Although secukinumab was associated with faster normalisation of genes (46% versus 13% of genes with >75% improvement at Week 4, respectively; p<0.05), levels of normalisation were similar by Week 24 (80% and 84% in the secukinumab and guselkumab groups, respectively). Additionally, guselkumab was associated with the normalisation of, three times more genes at Week 24 versus secukinumab (383 and 124, respectively, with >50% improvement and >25% treatment difference). Guselkumab also exhibited significant downregulation of the IL-23 receptor at Week 24 (p<0.05 versus baseline). At the cellular level, guselkumab was associated with a greater reduction of the disease-sustaining CD8+ tissue resident memory cells in psoriatic lesions versus secukinumab (p<0.05 at Week 24), while maintaining between Week 0 and Week 24 the frequency of suppressive regulatory T cells (which were reduced between Week 0 and Week 24 in the secukinumab group; p<0.05), leading to a higher ratio of regulatory T cells: CD8+ tissue resident memory cells in the guselkumab group.17 This is suggestive of a more favourable immune microenvironment and may at least partly explain the long-term efficacy characteristic of IL-23 inhibition, including tolerance of treatment withdrawal observed in some patients.
Taken together, the long-term disease control achieved by IL-23 inhibitors can be expressed in many ways, in particular the surrogate measure of infrequent dosing,1 superiority of response,4-6 and the slow loss of response following drug withdrawal.10-12 Each of these may be explained by the depth of inflammatory suppression and normalisation of skin phenotype provided by the unique mechanism of action of this drug class.17
Perspectives from Rheumatology: Going Under and Beyond the Skin
Daniel Aletaha
Aletaha moved from the skin to the joints, with an examination of the impact of IL-23 inhibition in patients with PsA. PsA is a heterogeneous systemic disease characterised by a multitude of musculoskeletal and skin manifestations. The majority of patients present with peripheral arthritis and/or psoriasis, with dactylitis and enthesitis reported between one-third to one-half of patients. Additional manifestations include spinal involvement, inflammatory bowel disease, and uveitis.18-23
Despite delayed diagnosis of PsA correlating with poor patient outcomes, the average time from symptom onset to diagnosis of PsA is 5 years. This highlights a need for greater awareness regarding the importance of early referral and timely treatment.24,25 Approximately one–third of patients with psoriasis eventually develop PsA and there is evidence that aberrant activation of the IL-23/IL-17 axis may play a key role in driving this transition, alongside genetic and environmental factors, as well as acting as a key upstream regulator of disease pathology.26,27
The Phase III DISCOVER-1 and -2 trials examined the safety and efficacy of guselkumab (100 mg q4w or 100 mg at Weeks 0, 4, and then q8w) versus placebo (with a switch to guselkumab at Week 24) in patients with active PsA despite standard therapies who were biologic-naïve (DISCOVER-2) or TNF inhibitor (TNFi)-experienced (DISCOVER-1; approximately 30% patients TNFi-experienced). ACR20 at Week 24 was achieved by over 50% of patients who received guselkumab in DISCOVER-1, and by over 60% in DISCOVER-2, regardless of guselkumab dosing in both trials, which was significantly greater than the placebo groups.28,29 Furthermore, rates of ACR20, 50, and 70 responses continued to increase from Week 24 in DISCOVER-2, with high response rates maintained through Week 100 across the dosing regimens (Figure 2),2 and during DISCOVER-1 from Week 24 to Week 52.30 This ACR response was also mirrored during the DISCOVER-2 trial with PASI 90 response, with over 60% patients achieving PASI 90 at Week 24 that was maintained through Week 100.2
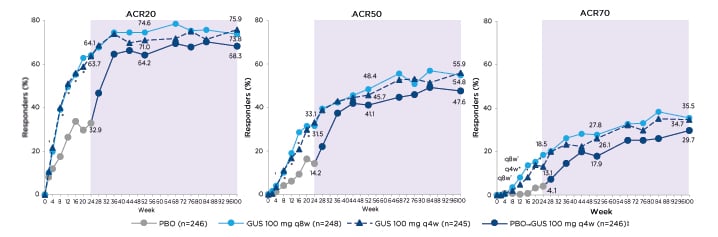
Figure 2: American College of Rheumatology responses in the DISCOVER-2 trial.
*p≤0.001
†p<0.05.
‡Includes randomised patients who received at least 1 dose of guselkumab.
Placebo-controlled period Week 0–24. Active-treatment period Week 24–100 (shown in purple shading).
Adapted from McInnes I et al.2
ACR: American College of Rheumatology; GUS: guselkumab; PBO: placebo; q4w: every 4 weeks; q8w: every 8 weeks.
Similar outcomes have been reported for other IL-23 inhibitors; namely risankizumab, which led to ACR20 and PASI 90 response rates of 51.3% and 55%, respectively, at Week 24 in patients with prior biologic experience in the KEEPsAKE-2 trial,31 and tildrakizumab, which led to high rates of ACR20 at Week 24 across a range of dose regimens during a Phase IIb trial (NCT02980692)32 in patients with or without prior use of TNFi (capped at 30% experienced) and naïve to IL-17, IL-12/23, and IL-23 inhibitors, which were maintained through 1 year, with a greater proportions of patients also achieving PASI 90 responses that continued to rise through 1 year.33
The clinical outcomes reported in DISCOVER-2 correlated with significant improvements in work productivity and daily activity through Week 52 in patients who received guselkumab versus placebo. Furthermore, active employment among patients unemployed at baseline increased by 10% following 1 year of guselkumab treatment.34
The Phase III COSMOS trial examined the impact of guselkumab (100 mg at Weeks 0, 4, and then q8w) versus placebo in TNFi-refractory patients with PsA. A significantly higher ACR20 response rate was noted as early as Week 4 in the guselkumab group versus placebo, with 44.4% versus 19.8% achieving ACR20 at Week 24, respectively (p<0.001). This increased numerically to 57.7% in the guselkumab group at Week 48. Similarly, ACR50 response rates increased numerically from 19.6% at Week 24 to 39.2% at Week 48 in the guselkumab group. This pattern was also reflected in PASI 100 response rates, which increased numerically from 28.6% at Week 16 to 53.4% at Week 48. Physical function, as reported by the Health Assessment Questionnaire Disability Index (HAQ-DI), was also significantly improved following treatment with guselkumab Week 24, with a mean change of -0.18 in guselkumab group versus -0.01 in the placebo group (p=0.003). HAQ-DI scores also continued to increase numerically from Week 24 to Week 48 in the guselkumab group, with a mean change of -0.40 at Week 48, whilst patients who crossed over from placebo to guselkumab at Week 24 achieved a mean change of -0.25 in HAQ-DI score at Week 48.35
It is also reassuring to note that a pooled analysis of >2,000 patients across four guselkumab clinical trials (VOYAGE-1 and -2, and DISCOVER-1 and -2) demonstrated stable rates of gastrointestinal-related serious AEs, with no new safety signals through 1 year of guselkumab treatment. Rates were comparable with placebo (during the placebo-controlled phase) and across patients with PsA during the DISCOVER trials, and patients with psoriasis during the VOYAGE trials.36
The data presented clearly demonstrate that IL-23 inhibition offers patients with PsA the possibility of improved outcomes across a range of assessments with a favourable risk-benefit profile for gastrointestinal-related serious AEs.
Putting the Pieces Together: Looking for Best Value in Psoriasis
Jo L.W. Lambert
In the final section of the symposium, Lambert discussed the need to evolve traditional models of healthcare by integrating self-monitoring and team-based care within specialised integrated practice units to improve the management of chronic diseases through a more personalised approach. New technologies offer novel methods of engaging patients and refocusing healthcare in such a team-based, patient-centred manner.37 Value-based healthcare is a paradigm that aims to improve patient outcomes without increasing costs, based on the definition that value is equal to outcomes and costs. This practice integrates key concepts such as multidisciplinary teams with medical leadership, outcome measures, and patient–doctor relationships, all backed up by a digital platform, to eliminate some of the underlying issues in current healthcare practices.38 The benefits of such an approach are multifactorial, and encompass multiple stakeholders, from better outcomes and lower costs to patients, higher rates of satisfaction, and efficiencies for healthcare providers to an alignment of prices with outcomes for suppliers, reduced healthcare spending, and improved health across societies.39
Outcome tracking and measurement is a key concept of value-based healthcare, acting as an improvement loop to optimise processes and treatment. The implementation matrix was developed as a roadmap to facilitate outcome tracking and can be applied across healthcare systems. The medical condition represents the matrix core and is surrounded by five dimensions: partnering with an internal core team and external collaborators; recording data and facilitating appropriate access within a data platform; comparing outcomes across providers to establish benchmarks; rewarding with investment and incentives; and improving through community learning and research.40
An appropriate integrated practice unit is organised around patient needs, provides a full cycle of care that includes patient education and follow-up, and involves a team of clinical and non-clinical personnel who are largely dedicated to the medical condition.41 Several steps are required to assemble a successful integrated practice unit, beginning with defining the condition and patient needs throughout the cycle of care. Identifying the mechanisms required to meet patient needs will help to gather and mobilise the most appropriate multidisciplinary team to provide clinical and patient support that also covers the wider context of comorbidities and complications, ultimately maximising value for the patient.41
Taking psoriasis as an example, such an approach incorporates screening for multiple conditions beyond the skin, including hypertension, diabetes, and PsA,42 with rapid access to partnering specialists as appropriate.41 Furthermore, a treat-to-target approach is increasingly being explored in dermatology, as illustrated by a recent Belgian consensus on the ideal treat-to-target outcomes for psoriasis management. This algorithm incorporates nine physician- and patient-reported outcomes across four domains (disease control, patient well-being, the burden of treatment, and manifestations beyond the skin), thereby supporting collaborative decision making between patients and doctors, and lending itself to integration within a value-based healthcare model (Figure 3).43
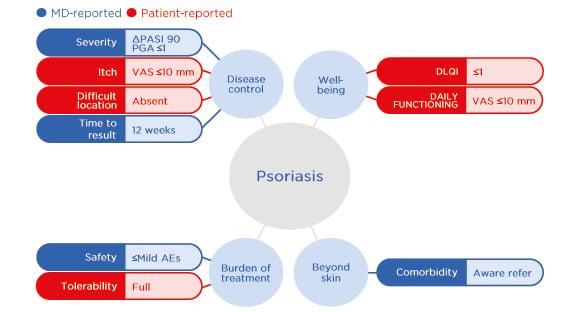
Figure 3: The Belgian treat-to-target ideal outcomes for psoriasis management.
Adapted from Grine L et al.43
AE: adverse event; DLQI: Dermatology Life Quality Index; MD: Doctor of Medicine; PASI: Psoriasis Area Severity Index; PGA: Physicians Global Assessment; VAS: visual analogue scale.
The importance of patient involvement is underscored by an ongoing Delphi study into defining ‘freedom from disease’ in patients with psoriasis, in which disparities were identified between the priorities of patients and doctors, in particular within psychosocial domains.44 Such insights are vital to inform appropriate treatment goals within a collaborative framework of decision making.
Data recording and exchange underpin the success of value-based healthcare, offering a more holistic approach to disease management, thereby optimising clinical decision making in a timely manner. There is, therefore, a need to overcome existing barriers to data exchange and promote coordinated, collaborative healthcare systems, with the aim of improving the experience of both patients and doctors.45 Technologies such as PsoSmart (Universiteit Gent, Belgium), an example provided by Lambert that is used in her hospital in Ghent, Belgium, are now being adopted, which encompass and centralise patient services (such as education, booking, and preparation), monitoring and assessing outcomes within treatment algorithms, and wider analytics where data are aggregated onto platforms for benchmarking and research.
Optimising outcomes also requires a minimisation of waste throughout the healthcare system, along with a reinvestment of savings. Eight key areas have been identified, with their associated waste-minimising actions: reduction of waiting/idle time; minimisation of inventory; eradication of defects; improvement in patient flow/transport; prevention of injuries and reducing motion; minimisation of overproduction; reduction of over-processing; and maximisation of human potential. These principles can be applied throughout healthcare systems and integrated practice units to minimise waste within an ongoing process of improvement, with home monitoring and task delegation particularly relevant to everyday practice.46
The potential benefits of screening and early intervention for patients with psoriasis are far-reaching. Early intervention with systemic or biologic therapies alongside wider screening has the potential to modify the disease course, reduce impairment and comorbidities, and reduce overall healthcare costs.47,48 Applying value-based healthcare through integrated practice units for psoriasis management will serve to optimise the already proven benefits of such approaches. Framing the medical condition as the focal point within a wide collaborative network will foster relationships, enhance the wider use of data, and ultimately maximise value for patients on a broader scale.
Summary
IL-23 is established as a key driver in the pathology of psoriasis and PsA, and there is clinical rationale for targeting the IL-23/Th17 signalling axis. Indeed, high rates of maintained PASI and ACR responses have been reported in patients with psoriasis and PsA who received treatment with guselkumab or other IL-23 inhibitors, and head-to-head trials in patients with psoriasis have confirmed the superiority of targeting IL-23 over alternative cytokines, with guselkumab demonstrating superiority compared to adalimumab for PASI 75 and PASI 90 at Week 16, and guselkumab demonstrating superiority compared with secukinumab for PASI 90 at Week 48. Furthermore, the clinical outcomes associated with IL-23 inhibitors are accompanied by improvements in patient-reported outcomes and a favourable risk-benefit ratio. The impact of IL-23 inhibition may be explained by the deep suppression obtained by upstream inhibition of the IL-23/Th17 signalling axis, a mechanism that appears to have a normalising action on gene expression within the psoriatic transcriptome.
With the potential for long-term disease control now a reality, it is key that this is optimally applied in a timely fashion within clinical practice. Adoption of value-based healthcare models and the use of integrated practice units will help increase awareness among healthcare practitioners. This will also promote the earlier diagnosis and treatment of patients, and such practices will not only drive improved outcomes for a particular condition, but also within the context of associated comorbidities and, with enhanced data sharing, society as a whole.
Date of preparation: November 2021
CP-242637







