Meeting Summary
Registries provide very high-quality data on the persistence of different therapies in the real world and can be used to compare and guide therapeutic guidelines. Dr Warren gave an overview of the different types of registries that capture data on patients with psoriasis. Furthermore, he discussed findings from the British Association of Dermatologists Biological Interventions Register (BADBIR), the Psoriasis Longitudinal Assessment and Registry (PSOLAR), and the Danish Biologic Interventions Registry (DERMBIO) data that help to gain insight on how best to prescribe drugs in a clinical setting. Prof Puig and Prof Gniadecki presented cases encountered in clinical practice to illustrate how real-world data can support the clinical decision-making process. Throughout their presentations, Prof Puig and Prof Gniadecki engaged the audience in interactive discussion on how to improve patient monitoring and management of comorbidities, and addressed issues such as drug survival, safety, and economics.
Interpreting Long-Term Registry Data in the Treatment of Psoriasis
Doctor Richard Warren
The presentation compared registries used to capture data in patients with psoriasis, evaluated the differences between studies that use registry data, and provided insights into how registry data should be interpreted.
Real-world evidence (RWE) is used to evaluate the impact of treatments in a routine clinical setting. RWE can be obtained from various sources, including patient registries, existing electronic health records, routinely collected administrative data, primary patient data collection, and/or population surveys. Registries can provide information about a disease and/or therapeutic strategies. Compared with randomised controlled trials (RCT), RWE offer many advantages. Of note, RCT are commonly driven by an efficacy endpoint and are seldom powered to look at safety either in detail or the long term. Furthermore, the professional support networks that exist within RCT are often not available in a real-world setting, which can impact outcomes including treatment adherence, persistence (the duration of time from initiation to discontinuation of therapy),1 and efficacy.
There are currently three key styles of registries for psoriasis and psoriatic arthritis (PsA): pharmacovigilance registries, epidemiology or observational studies, and network registries. Examples of pharmacovigilance registries include BADBIR, the German Psoriasis Registry (PsoBest), DERMBIO, and PSOLAR. BADBIR is a prospective observational comparator registry for patients with moderate-to-severe psoriasis receiving biologics (n=8,424) or conventional systemic therapies (n=4,488) and collects data from 151 dermatology departments across the UK and Ireland. An early publication based on these data compared the baseline characteristics of the patients between two cohorts (biologics [n=5,065] and systemic therapies [n=3,334]).2 The findings showed that patients who had psoriasis for a reasonable amount of time (mean disease duration: 23.0±12.6 years versus 19.0±13.4 years) and those receiving biologics compared with non-biologics were generally heavier (mean body weight: 90.3±21.5 kg versus 87.2±21.4 kg).2 All patients demonstrated high Psoriasis Area Severity Index (PASI) scores (16.4±8.3 versus 15.5±7.9, respectively), which were reasonably well matched between the cohorts.2 A 5-year follow-up allowed the assessment of treatment, disease activity, and adverse events (data not shown).3
PsoBest is a registry for patients with moderate-to-severe psoriasis, with and without arthritis, with a 5-year observation time and follow-up of every 3 months; patients from this registry were treatment-naïve receiving biologics or non-biologic systemic therapies. Patients receiving biologics in this registry had a significantly greater mean duration of disease compared with systemic therapy (21.9±14.1 years versus 16.9±0.0 years). All other baseline characteristics were well matched.4,5 PsoBest provided additional value in that it collected data on the first-line systemic therapy Fumaderm®, and therefore may provide RWE on the use of fumerates in these patients.
DERMBIO is a registry for patients with psoriasis vulgaris receiving biologics (adalimumab [n=576]; etanercept [n=176]; infliximab [n=176]; ustekinumab [n=170]) with a 10-year data collection period.6 Although this registry does not have a conventional therapy cohort, it provides other valuable RWE, for example, it shows that patients prescribed adalimumab, etanercept, or infliximab are more likely to have PsA compared with patients prescribed ustekinumab (38.1%, 39.6%, or 43.8% versus 14.1%, respectively).6 Furthermore, patients prescribed the former three treatments are also more likely to be receiving concomitant methotrexate (21.9%, 22.5%, or 55.1% versus 12.4%, respectively).6 Therefore, registries such as DERMBIO add value by helping to capture prescribing habits in a real-world setting.
PSOLAR is a registry for patients with moderate-to-severe psoriasis from 300 practices across North America, Latin America, and Europe. More patients in this registry receive biologics (ustekinumab, n=4,364; infliximab, n=1,394; or other biologics, n=4,251) compared with systemic therapies (n=2,804).7 The value of this registry in providing RWE is highlighted in the vast patient numbers.
There are several key factors to consider when interpreting data obtained from registries: the size of the registry and/or whether the registry is powered to address the investigative question of interest. In addition, the external validity of the registry is important; for example, BADBIR collects data nationwide, indicating it is a reliable representation of the real-world setting in the UK and Ireland. Two other important factors of consideration are whether an a priori question was set and what adjustments have been performed.
Data from the PSOLAR registry in 2016 demonstrated that ustekinumab (n=1,041) was the most effective treatment compared with infliximab (n=116), adalimumab (n=662), and etanercept (n=257) over 12 months, measured by the proportion of patients with a Physician’s Global Assessment (PGA) score of 0/1 (Figure 1).8 Adjusted logistic regression analyses demonstrated that patients receiving tumour necrosis factor (TNF)-α inhibitors were significantly less likely to achieve a PGA score of 0/1 at 6 months compared with ustekinumab patients. Similar estimates were observed at 12 months, but only the infliximab versus ustekinumab data were significantly different.8 In view of efficacy measures based on registry data, it is important to emphasise that PGA or PASI data are often missing. Accordingly, investigative efficacy questions may not always be possible or provide the full efficacy assessment. Consequently, drug persistence may be used.
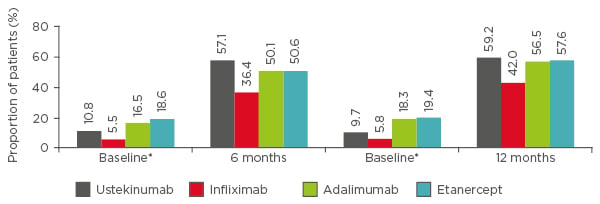
Figure 1: PSOLAR data. Proportion of patients with Physician’s Global Assessment score of 0 (clear) or 1 (minimal) at 6 and 12 months.
Data were analysed from 2,076 users who initiated infliximab (n=116), adalimumab (n=662), etanercept (n=257), or ustekinumab (n=1,041) during PSOLAR participation. Only the first biologic started during registry participation was analysed. Of the participants, 80% had been exposed to a biologic prior to enrolment. Evaluations were limited to patients who had baseline data and continued their initiated therapy at their 6-month and/or 12-month visits.
*Baseline psoriasis severity was assessed at the closest visit before the first dose of the newly initiated biologic.
PGA: Physician’s Global Assessment; PSOLAR: Psoriasis Longitudinal Assessment and Registry.
Adapted from Strober et al.8
Persistence is an important parameter in the measure of long-term therapeutic performance in a real-world setting6 and arguably an appropriate surrogate for how successful a drug may be in a given population over time. Bio-CAPTURE,9 a small registry based in the Netherlands collecting data across eight regional, non-academic centres, showed ustekinumab (n=66) to have the highest long-term drug survival compared with adalimumab (n=101; p=0.066) and etanercept (n=82; p=0.032) in 2011. A Japanese registry also demonstrated ustekinumab to have the highest long-term drug survival compared with infliximab (n=38) and adalimumab (n=59); both of which had a significance of p<0.05.10 However, in view of earlier comments regarding registry size the small patient numbers in the registry would suggest these findings should be interpreted tentatively. Shortly after the Japanese and Dutch study data were released, data from the PSOLAR registry indicated ustekinumab to have the highest persistence compared with infliximab, etanercept, and adalimumab as a first, second, and third-line biologic.11 Although taking into consideration that PSOLAR is a single company-sponsored registry, this conclusion may also be only tentatively accepted by the clinical community. BADBIR, however, corroborated these findings over a 3-year follow-up and overcomes concerns regarding patient numbers and single company-sponsored registries.12 Altogether, the similar findings across different registries confirm the validity of the findings. Accordingly, these data support ustekinumab as the gold standard for psoriasis treatment in terms of persistence; however, recent RCT have indicated interleukin (IL)-17 inhibitors to be superior to ustekinumab.13 If these findings translate into a real-world setting, IL-17 may be considered as a potential treatment over the coming years.
RWE from registry data have provided insights into the most common reasons for drug discontinuations. Data from BADBIR indicated ineffectiveness of therapy, particularly etanercept, and adverse events, particularly infliximab, to be the most common reasons for patients discontinuing biologics, while PSOLAR identified ineffectiveness to be the primary reason.11 Data from PsoBest suggest biologics to be associated with a high rate of serious infections compared with conventional systemic non-biologics.5 In consideration of the factors discussed earlier when interpreting data from registries, PsoBest may not have been powered sufficiently to address investigative questions around serious infections. Both BADBIR and PSOLAR reported infections to be more common with infliximab use compared with other biologics. The current trend to use infliximab less often in real-world settings may be a reflection of the latter.
In conclusion, the data presented suggest that RWE based on registry data will be beneficial for long-term monitoring of adverse events and efficacy in psoriasis patients. Through future collaborations and publications of RWE, we will also continue to improve the wealth of information available in the field of dermatology.
Real-Life Experiences and Clinical Cases: An Interactive Discussion
Professor Lluís Puig and Professor Robert Gniadecki
As the need to provide a personalised treatment approach is becoming more important, the demand for real-world data to aid clinical decision-making in the treatment of psoriasis is increasing. Prof Puig and Prof Gniadecki presented patient cases to illustrate how real-world data can support clinical considerations such as patient adherence, comorbidities, drug survival, patient monitoring, safety, and economics.
Case 1: Association Between Psoriasis and Inflammatory Bowel Disease
A 25-year-old breastfeeding woman presented with mild psoriasis, which she had for the past 14 years, a PASI of 11.3 with no PsA, a history of intermittent abdominal pain, and occasional diarrhoea without blood in the stool. In light of these symptoms, clinical decision-making was centred around screening the patient for inflammatory bowel disease (IBD) or therapy with anti-IL-17 agents, which would likely exacerbate the IBD.
An analysis of data from DERMBIO, a Danish nationwide cohort study of 5.5 million patients,14 found a psoriasis-associated increased risk of Crohn’s disease and ulcerative colitis that was higher in severe psoriasis. Additionally, an increased risk of psoriasis in patients with IBD was also observed. However, of the 11,000 patients with Crohn’s disease and the 30,000 patients with ulcerative colitis combined, only 82 had mild psoriasis and 54 had severe psoriasis, potentially explaining why clinicians rarely encounter this combination of conditions in practice.
Faecal calprotectin measurements are routinely used in the diagnosis and monitoring of patients with IBD; however, given the risk of obtaining false-positive results with this method,15 the patient should ideally be referred to a gastroenterologist. The patient denied colonoscopy and referral to a gastroenterologist but noticed that her symptoms improved on a gluten-free diet. The patient was tested for coeliac disease and tested positive for anti-transglutaminase antibody. Although a meta-analysis comparing real-life data to registry data suggested an association between coeliac disease and psoriasis,16 the link between a gluten-restricted diet and improvement in psoriasis is still lacking. Eventually, the patient was seen by a gastroenterologist who was unable to make a final diagnosis; after 3 months on a gluten-free diet, her psoriasis improved.
Case 2: Paradoxical Onset of Psoriatic Arthritis?
A 39-year-old man with psoriasis for the last 15 years, who was given methotrexate 15 mg on psoriasis flare, had to discontinue treatment after 2 months due to lack of efficacy and increased liver transaminases. The patient had a PASI of 10.6 before initiation of ustekinumab 90 mg (given at Week 0, Week 4, and then every 12 weeks). After 4 months of treatment, his PASI decreased to 1.2; however, after 11 months of treatment, the patient developed joint pain, three tender joints, one swollen joint, and occasional knee pain. No radiographic changes were detected and the patient was referred to a rheumatologist, who diagnosed possible PsA as a result of ustekinumab treatment. Clinical considerations for this patient included the potential for the joint pain being a treatment-related adverse event and impacted the decision to continue or discontinue treatment with ustekinumab.
A retrospective study assessing patients with psoriasis receiving biologic treatment found that 22 out of the 327 patients who met the inclusion criteria developed PsA during treatment: 6 (27.2%) patients who received etanercept therapy, 10 (45.4%) who received adalimumab, 4 (18.2%) who received ustekinumab, and 2 (9.2%) who received infliximab.17 These results suggest that biologic therapy may not be sufficient to prevent the onset of articular involvement, and in most of the verified PsA cases, arthritis occurred in concomitance with severe cutaneous involvement.
Therefore, it is possible to develop PsA-like symptoms and signs on treatment with both TNF-blockers and ustekinumab, and a diagnosis of paradoxical arthritis was ruled out. In this instance, owing to a lack of history of joint pain, the inflammation status of the joint was determined, and the patient was given an intra-articular injection to allow the continuation of ustekinumab treatment, which ultimately improved the patient’s skin.
Case 3: Dose Escalation
An obese 68-year-old man with a 30-year history of psoriasis, no PsA, mild hypertension, and a daily smoking habit, was previously treated with methotrexate and adalimumab but discontinued both due to lack of efficacy. The patient was started on adalimumab 40 mg every other week and had a good response (PASI 0–6) for 3 years. Upon psoriasis flaring up the dose was increased to 40 mg every week. As a result of these flare ups clinicians considered the options of continuing the patient on a higher dose, reverting to the standard dose of 40 mg every other week, or changing the biologic treatment entirely.
In an unpublished dose-escalation study by Gniadecki,18 1,256 patients receiving 40 mg adalimumab every other week achieved PASI ≥75 (64.1%), PASI ≥90 (40.3%), and PASI 100 (21.7%). The 349 (27.8%) patients who had a PASI <50 during Weeks 24 and 252 of the study were dose- escalated to 40 mg every week. Of these 349, 182 (52.1%) remained on every-week dosing and 167 (47.9%) achieved a PASI 75 response and were de-escalated to every other week. Later, 83 patients were re-escalated to every-week dosing, owing to a PASI <50 response (Figure 2).18,19
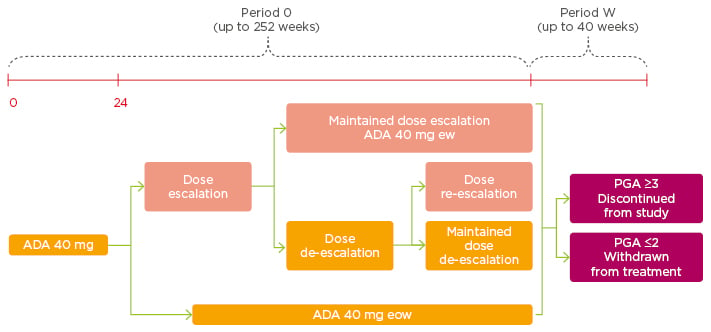
Figure 2: Dose escalation.
Adalimumab 40 mg every week can be considered in patients with inadequate response to adalimumab 40 mg every other week.
ADA: adalimumab; eow: every other week; ew: every week; PGA: Physician’s Global Assessment.
Adapted from Gniadecki18 and Gniadecki et al.19
After escalation of adalimumab dosing to every week, approximately one-quarter of patients were able to successfully have their dose de-escalated and remained on every-other-week dosing for nearly 1 year without the need for dose re-escalation. Therefore, transient increase in the dosing frequency of adalimumab to every week improved responses to treatment and permitted long-term maintenance to be achieved.
Unmet Needs
There are a number of unmet needs in the clinical management of psoriasis. Focus on real-world practice needs to increase by moving away from population measures, such as PASI 75 and PASI 90, which are best suited to drug comparison in clinical trials, and moving towards individual measures (e.g., absolute PASI). Also, steps need to be taken to avoid inequality of care and ensure that access to treatment is improved for certain patient populations, such as elderly female patients with low educational and socioeconomic status.
PASI, the most frequently used clinical severity scale in clinical trials and drug approval, often depends on a 75% improvement in the baseline PASI score. In clinical trials, the mean baseline PASI is 20, whereas in real life it is closer to 12 or lower; therefore, the true success of psoriasis treatment seems to be under-represented. This discrepancy may relate to the way numerical values are assigned to the degree of body surface area involvement; thus, a better method to assess clinical improvement is needed.
Moreover, the relevance of baseline PASI diminishes with increasing duration of treatment, which implies that absolute PASI values are more appropriate to assess long-term response. Absolute PASI scores can be used where PASI 2 corresponds to a PASI 90, and a PASI 5 score, often considered the threshold for therapeutic adjustment or switching, corresponds to a PASI 75 or better response.20
Economics and Adherence
In the first year of treatment, some biologics carry a significant increase in their dose and, consequently, their cost. Therefore, it might be more sensible to switch to a drug that has a relatively small increment in the induction phase, rather than to others that might have a larger increment. Puig et al.21 developed a decision tree with a 2-year time horizon to compare the cost consequence of biologic drugs for moderate-to-severe psoriasis from the perspective of the Spanish National Health System. Secukinumab monotherapy was found to be associated with the lowest cost per responder, followed by infliximab, and then ustekinumab.
Low adherence to therapies in psoriasis decreases treatment outcomes and increases total healthcare costs. Hsu and Gniadecki22 surveyed patients’ attitudes to treatment and measured adherence to biologics using the medication possession ratio index in a population of patients treated for psoriasis vulgaris. The medication possession ratio was calculated based on hospital records documenting the dispensing of biologics to patients, PASI, Dermatology Life Quality Index, presence of PsA, concomitant treatment, and cause for treatment discontinuation, all of which were obtained from DERMBIO. Patients’ attitudes and beliefs were measured using the Medication Adherence Rating Scale. The authors found that adherence to biologics was very high, which is consistent with a positive attitude to treatment.
Factors Impacting Drug Survival
Biologic drug survival in psoriasis reflects long-term performance in real-life settings.23 In economies where there is a need for sustainability, it is common practice to optimise the treatment dose by lengthening the dosing intervals for patients achieving PASI 90 and PASI 100. Sex, obesity, comorbidities, previous biologic exposure, and combination treatment are some of the variables that affect PASI response.23 A retrospective, observational study on biologic drug survival in a real-life cohort of patients with moderate-to-severe chronic plaque psoriasis, found that cumulative probability of drug survival was lower in obese patients and significantly higher for ustekinumab than for any other biologic agent.23 Multivariate analysis showed that obesity, etanercept treatment, and strict adherence to approved doses were associated with an increased probability of drug withdrawal, whereas ustekinumab treatment, and PASI 75 and PASI 90 responses at Week 16, prolonged drug survival.
Patients with Comorbidities
A 67-year-old male who has had psoriasis for 10 years, with no PsA, had a PASI of 16.4. The patient was obese, had unstable angina and hypertension, and eventually suffered a myocardial infarction. He had been subject to many treatments with no response; thus, the first treatment choice was an anti-TNF agent or methotrexate because both drugs are associated with a reduced rate of cardiovascular events. Cyclosporine should be avoided in this patient type due to the resulting increase in blood pressure and vascular resistance.
A 57-year-old woman with latent tuberculosis received adalimumab before starting on a efalizumab. After 22 months on adalimumab, she presented with pneumonia. In elderly populations, and particularly vulnerable patients, a pneumococcal vaccine is prudent as these individuals are at a high risk of infection. A study by Dommasch et al.24 examined the risks of infection and malignancy with the use of TNF antagonists in adult patients with psoriatic disease and found that there was a small increased risk of overall infection with the short-term use of TNF antagonists. Of reported infections, 97.6% were non-serious, and the large majority of these were upper respiratory tract infections.
A 67-year-old woman on infliximab acquired a left knee prosthesis infection, so her treatment was stopped. Following psoriasis relapse, she was started on ustekinumab and the infection was initially controlled with antibiotics. However, because her condition had become unstable and the prosthesis was replaced, a decision had to be made about the interruption of biologics prior to her operation. A study by Bakkour et al.25 looked at the risk of postoperative complications in patients with psoriasis on biologic therapy undergoing surgical procedures. The authors reported that continuing biologic therapy in patients with psoriasis and PsA peri-operatively did not increase the risk of postoperative complications, and that interrupting biologic therapy peri-operatively significantly increased the risk of disease flare. There are currently very little data on the subject and for minor surgery. Some practitioners are stopping biologic therapy, which could have a detrimental effect on patients’ psoriasis. However, for major surgery the risk of infection changes; therefore, stopping treatment should be considered and approached on a case-by-case basis.







