Meeting Summary
Prof Reich outlined our latest understanding of relevant psoriasis pathophysiology. Psoriasis was believed to be a skin disease mediated by T helper cell 1 (Th1 cell) 20 years ago; it has now been shown to be driven by Th17 cells, which are stimulated by a number of proinflammatory cytokines, among which IL-23 is overexpressed. Characteristics of the individual antibodies determine clinical properties. IL-23 inhibitors have long injection intervals, and inhibit more regulatory than effector cytokines.
Prof Strober reviewed key clinical data on IL-23 inhibitors including that from VOYAGE 1 and 2, NAVIGATE, and ECLIPSE for guselkumab; reSURFACE 1 and 2 for tildrakizumab; and ultIMMa-1/2 and IMMvent for risankizumab. Taken together, the many comparator studies suggest that the IL-23 inhibitors deliver robust and long-lasting efficacy, with long treatment intervals and with relative safety; there are few contraindications to use an IL-23 inhibitor. Prof Strober said he believes that, over time, this class will replace ustekinumab and become the first-line therapeutic approach in psoriasis.
Prof Conrad gave an overview of patients’ needs and the drug, patient, and disease-related factors to be considered when choosing a therapy from the increasing numbers available. He stressed that no single agent or class is appropriate for all patients and that, in many instances, traditional anti-TNF are being superseded in terms of both efficacy and safety by newer drugs. Data on some disease-related factors, e.g., the presence of psoriatic arthritis, however, support the use of anti-TNF. Prof Conrad outlined his considerations regarding drug choice for patients with conditions such as pregnancy, inflammatory bowel disease (IBD), latent tuberculosis (TB), or hepatitis B virus (HBV).
Introduction
With increasing treatment options in psoriasis (e.g., TNF inhibitors, IL-12/IL-23 inhibitor ustekinumab, IL-17 inhibitors, and now IL-23 inhibitors), treatment strategies have become increasingly complex. This symposium aimed to help physicians manage this complexity and consider how advances in treatment can be used in the clinic to ensure that individual patients are offered the most appropriate therapy.
Navigating the Complex Waters of Psoriasis Treatment: Assessing the Best Target
Professor Kristian Reich
For many years, psoriasis was considered to be a skin disease mediated by Th1 cells.1 TNF is a signature cytokine of the Th1 response, and therefore anti-TNF therapies, such as etanercept,2 infliximab,3 and adalimumab,4 were introduced in the early 2000s. Ustekinumab,5 which inhibits both IL-12 and IL-23, was introduced in 2009. The IL-17 blockers secukinumab,6 ixekizumab,7 and brodalumab8 (anti-IL-17R) came next, followed by IL-23 inhibitors guselkumab,9 in 2017, and more recently, tildrakizumab10 and risankizumab.11
Patients have individual patterns of psoriasis and disease domains or manifestations include the scalp, palmar-plantar, pustular versus nonpustular, nail disease, psoriatic arthritis, and pericardial disease. Specific cytokine pathways may contribute differentially to various disease domains; this may influence the choice of therapy.
The pathophysiology of psoriasis involves epidermal hyperproliferation, abnormal differentiation of epidermal keratinocytes, and decreased keratinocyte apoptosis, all contributing to a thickening of the epidermis. An influx of immune cells, particularly T cells, is the main driver of psoriasis pathophysiology. Histological samples have shown that the T cells and dendritic cells sit in direct proximity in the skin;12 they work in tandem and the so-called cross-talk between them is the focus of research interest in psoriasis. Immunologic cells, including the dendritic cells, release an ‘educational’ cytokine which activates T cells and leads to the development of Th1 and/or Th17 cells. Ten years ago, it was believed that IL-12 stimulated the overexpression of Th1 cells in psoriasis;13 now it is believed that overexpression of the Th17 pathway, stimulated by IL-23, is the key driver in this disease.
Th17 cells release cytokines such as IL-17, which activate keratinocytes and initiate the epidermal pathology in psoriasis.14 Once activated, keratinocytes are themselves an active source of cytokine mediators that then signal back and modulate the immune system. Epidermally-derived cytokines such as IL-19, IL-1β, IL-36, and IL-17C are relevant targets for agents in development or future drugs. The system is modulated via feed-forward and feed-backward responses.
This simplified upstream/downstream model (Figure 1) demonstrates that dendritic cells produce TNF-α that, acting synergistically with IL-17, activates keratinocytes. The dendritic cells also produce IL-23 that, in turn, activates neutrophils and T cells to produce IL-17. The model does not, however, explain the potential downside of inhibiting IL-17. In some patients, IL-17 inhibition leads to exacerbation of IBD. This unexpected clinical finding led to the new understanding that Th17 cells may be pathogenic or nonpathogenic. Healthy skin has nonpathogenic Th17 cells that produce physiological IL-17 and protect against candida infection in the absence of IL-23. In the presence of IL-23, pathogenic Th17 cells overproduce IL-17.15,16 IL-17 inhibitors are only given to patients with elevated IL-17 levels to normalise them, but over-suppression may increase the risk of candida infections. This is not seen with IL-23 inhibition.
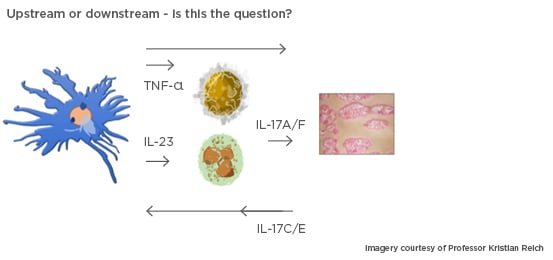
Figure 1: A simplified model demonstrating the importance of activation of dendritic cells in the epidermis.
This type of therapeutic molecule (e.g., human monoclonal antibodies [adalimumab] or polyethylene glycol PEGylated human fragment antigen-binding region [certolizumab]17) may have distinguishing properties in the clinic. The mechanism by which agents inhibit IL-17 also influences its clinical properties. Secukinumab and ixekizumab inhibit both the IL-17A homodimer and the IL-17A/F heterodimer. Bimekizumab, currently in development, inhibits IL-17A and IL-17F as well as the IL-17A/F heterodimer. Brodalumab has a wider effect, blocking its receptor, and thereby the signalling for IL-17A, IL-17F, IL-17E, and IL-17C.18
Selective targeting of IL-23 varies according to the agent. The IL-23 receptor is comprised of subunits p40 and p19: the IL-12 receptor of p35 and p40 subunits. Ustekinumab binds to p40, thereby inhibiting both IL-23 and IL-12. The IL-23 inhibitors guselkumab, tildrakizumab, and risankizumab selectively target the p19 subunit and inhibit IL-23, but not IL-12. IL-12 is now thought to have anti-inflammatory properties in psoriasis, playing a role in defence against intracellular pathogens, and inhibiting IL-23 alone has been shown to be preferable to inhibiting IL-12/IL-23.19
Differences between drugs in terms of pharmacokinetics, dose, immunogenicity, affinity, potency, and specificity are characteristic of the antibodies themselves; three antibodies with the same target may have different properties in practice. There are also class effects; the long injection intervals (every 8 or 12 weeks) with IL-23 inhibitors may be due to the drugs’ upstream effect (Figure 1), inhibiting an educational rather than an effector cytokine. Response rates are high and stable with this class of drug.
One reason why psoriasis can reappear once therapy is stopped could be that an inflammatory memory develops if the disease has been uncontrolled over time. Researchers have found that treatment with the IL-17A inhibitor secukinumab reduced counts of the so-called resident memory T cells,20 which carry the inflammatory memory. IL-23 inhibitors may have an even greater effect than IL-17 inhibitors on the resident memory T cells, but currently this is unproven. It has been shown that patients responding to guselkumab lose response only slowly if the drug is discontinued,21 and a proportion maintain response for a prolonged period without further treatment. Patients responding to guselkumab at Week 20 were randomised to continue treatment (n=193) or to receive placebo (n=182). Six months later, at Week 48, more than one third of the patients (36.8%) in the placebo arm continued to have a Psoriasis Area Severity Index (PASI) 90 response21,22 without being on active treatment. This observation needs to be explored further.
Clinical and biological markers associated with long-term maintenance of PASI 90 response following withdrawal of guselkumab23 include a range of factors such as lower BMI (p<0.001), PASI 100 response at Week 28 (p<0.005), and a shorter disease duration (p<0.005).23 Prof Reich said that allowing a patient’s disease to remain uncontrolled may reduce the chance of him/her ever becoming disease-free while not on active treatment.
What Do the Data Say? Evidence-Based Decision Making for Psoriasis Treatment
Professor Bruce Strober
In 2009, the approval of ustekinumab, which inhibits IL-12/IL-23, represented a significant advance in psoriasis therapy. The PHOENIX 1 study found that of 255 patients given 45 mg ustekinumab, 41.6% achieved PASI 90, compared to 2.0% on placebo (p<0.0001).24 In 2017, the specific IL-23 inhibitor guselkumab was compared with the anti-TNF-α agent adalimumab in the VOYAGE 1 study.25 Patients with moderate-to-severe psoriasis received placebo (n=174), guselkumab (n=329; 100 mg at Weeks 0 and 4 then q8w), or adalimumab (n=334; 80 mg Week 0, 40 mg Week 1, then 40 mg q2w). At Week 16, 73.3% of the guselkumab arm had achieved PASI 90, and this was maintained, with 76.3% of the group still having PASI 90 at Week 48. This compared to 49.7% of the adalimumab arm with PASI 90 at Week 16, dropping to 47.9% at Week 48.
Rates of PASI 90 in the guselkumab arm were approximately equivalent to rates of PASI 75 in the adalimumab arm.25 Prof Strober said this comparison puts the efficacy of guselkumab into perspective. Rates of PASI 100 in the guselkumab arm were approximately double those in the adalimumab arm (37.4% versus 17.1% at Week 16; 47.4% versus 23.4% at Week 48);25 patients had almost a one in two chance of having totally clear skin after a year of guselkumab therapy.
At Week 52, adalimumab nonresponders (failure to achieve PASI 90) in VOYAGE 1 were switched to guselkumab.26 At Week 100, 73% had achieved PASI 90 and 42% had achieved PASI 100,26 findings replicated in VOYAGE 2. The therapy switch turned inadequate responders into responders, Prof Strober said.
The NAVIGATE study27 included patients who did not achieve an Investigator’s Global Assessment (IGA) score of 0/1 after 2 doses ustekinumab (n=135). At Week 16, they were switched to guselkumab; those who were switched derived significant benefit over those were maintained on ustekinumab (n=133). Greater proportions achieved IGA 0/1 and at least a two-grade improvement at Week 28 (31.1% versus 14.3%; p=0.001) and at Week 52 (36.3% versus 17.3%; p<0.001).27
In the Phase III ECLIPSE trial,28 guselkumab and secukinumab were compared head-to-head. In the guselkumab arm (n=534), a 100 mg dose was given at Weeks 0, 4, 12, then q8w. In the secukinumab arm (n=514), a 300 mg dose was given at Weeks 0, 1, 2, 3, 4, then q4w. Initially, at Week 12, patients in the secukinumab arm were more likely to have a PASI 90 response (76.1% secukinumab patients versus 69.1% guselkumab patients). But over time, the situation reversed and at Week 48, the PASI 90 rates confirmed the superiority of guselkumab over secukinumab (70.0% secukinumab patients versus 84.5% guselkumab patients; p<0.001).28 The study used the conservative, nonresponder imputation (NRI) approach. Prof Strober said that the guselkumab arm showed a stable PASI 90 response rate, whereas the rate in the secukinumab arm declined slightly, implying that the gap between the arms may widen over time.
Tildrakizumab is another approved IL-23 inhibitor which specifically blocks the p19 subunit, however data from the reSURFACE trial29 suggests lower efficacy than is seen with guselkumab. The trial compared tildrakizumab with placebo or etanercept; at Week 12, 61% of patients receiving 100 mg tildrakizumab, and 66% of those receiving 200 mg tildrakizumab, had achieved a PASI 75 response. By Week 28, 74% patients on either tildrakizumab dose had achieved PASI 75 response. A PASI 90 response was achieved by 37% of patients on the lower dose and 39% of patients on the higher dose at Week 12. By Week 28, these figures were 56% and 58%, respectively. A PASI 100 response was achieved by 12% of patients on either dose at Week 12, and by 23% and 27% in the 100 mg and 200 mg groups, respectively, at Week 28.
Risankizumab is the third IL-23 inhibitor approved in moderate-to-severe psoriasis. In the ultIMMa-1 trial,30 75.3% patients receiving risankizumab achieved a PASI 90 response at Week 16, rising to 81.9% at Week 52. In the comparator group, 42.0% of those receiving ustekinumab achieved a PASI 90 response at Week 16,30 rising to 44.0% at Week 52. Some loss of response between injections was seen in the ustekinumab group, while the response with risankizumab remained stable between injection intervals. Integrated data from ultIMMa-1 and ultIMMa-230 showed that 88.4% of risankizumab patients who achieved PASI 90 at Week 16 retained the response at Week 52 compared to 73.3% receiving ustekinumab (p<0.001).
The IMMvent study31 compared risankizumab with adalimumab. At Week 16, patients randomised to risankizumab continued with this treatment; those on adalimumab with a PASI 90 response continued with adalimumab. However, nonresponders to adalimumab (PASI <50) were switched to risankizumab; those with intermediate response (PASI 50–<90) were rerandomised to either adalimumab or risankizumab. At Week 44, 76% of those who received risankizumab throughout the study had PASI 90 response. Among the intermediate responders who switched therapy, 66% achieved PASI 90 compared to 61% among nonresponders who switched. Of those rerandomised to adalimumab, 21% had PASI 90 at Week 44. This suggests that patients can be successfully switched from adalimumab to risankizumab, but those with an initial intermediate or nonresponse to adalimumab may be harder to treat.
The IL-23 inhibitors have a reassuring safety profile with few notable differences between the various agents. None are associated with serious infections, major adverse cardiac events, malignancy, or death. Patients in the guselkumab arm of VOYAGE 1 and VOYAGE 2 were followed for 156 weeks and no clear discrepancy was found in serious infections, malignancies excluding nonmelanoma skin cancers, major adverse cardiac events, or deaths.32,33 The safety profile of tildrakizumab is again reassuring. The higher dose of tildrakizumab was not associated with more infections than the lower dose, and there appeared to be a large therapeutic window within the limits of the doses used in moderate-to-severe psoriasis.
Data from the ultIMMa trials30 suggest risankizumab has a similar safety profile; upper respiratory tract infections account for most of the infections that occur more frequently.30 Analysis of pooled clinical trial data found a slight increase in fungal infections, predominantly superficial tinea infections, and a few cases of herpes zoster which did not necessitate discontinuation of treatment.34
Studies on the three IL-23/p19 inhibitors have included patients who tested positive for latent TB prior to entry and were allowed to receive prophylaxis with standard local regimens. There were almost no cases of reactivation of TB.9-11 None of the 105 patients with latent TB receiving guselkumab developed active disease: nor of the 103 receiving risankizumab. One patient developed TB while receiving tildrakizumab, but of the 55 with latent disease receiving prophylaxis, none developed active TB.
In conclusion, IL-23 inhibitors deliver robust and long-lasting efficacy even where dosing is infrequent and variable. In fact, patients can miss a dose by up to 3 weeks and still maintain the response. Taken together, the many comparator studies suggest that the IL-23 inhibitors provide better efficacy and equivalent safety to ustekinumab. Over time, this class is likely to replace ustekinumab and become the first-line therapeutic approach in psoriasis. The efficacy of IL-23 inhibitors is yet to be determined in both treating psoriatic arthritis and reducing systemic inflammation. Because there are few contraindications to the use of IL-23 inhibitors, patients with IBD, multiple sclerosis, liver disease, or heart disease may all be considered for these drugs.
Patient Profiling in Daily Practice: Which Drug for Which Patient?
Professor Curdin Conrad
There are >10 approved biological therapies for psoriasis which makes choosing the optimal therapy a complex decision. As an example, a recent publication35 demonstrated the potential importance of the choice of first biologic. Of patients with moderate-to-severe psoriasis who received the IL-17 inhibitor secukinumab as first-line therapy, approximately 80% were still taking the drug after 3 years. This figure fell to approximately 50% among those receiving secukinumab as the second-line biologic, and to approximately 30% among those receiving the treatment after two or more previous biologics.
There are no current guidelines stating which drug should be given to which patient. In making a choice, drug-related factors include efficacy, safety, and convenience which in turn affects compliance. Patient-related factors include age, sex, child-bearing potential, demand, or expectation. Disease-related factors include disease activity and course, the phenotype (whether there is nail psoriasis, palmoplantar psoriasis, or psoriatic arthritis), the severity and impact of the disease, and comorbidities. Taken together, these factors feed into drug survival; no single treatment is effective and appropriate for all patients.
On drug efficacy, as has been described, an early meta-analysis36 showed ustekinumab to be more efficacious than the anti-TNF therapies adalimumab and etanercept. The IL-17 inhibitors, approved in 2015, were associated with significantly higher response rates than ustekinumab;37 the introduction of IL-23 specific inhibitors was associated with still higher PASI responses and high levels of maintained efficacy.28
Regarding safety, meta-analyses show no major differences between anti-TNF therapies and anti-IL- 12/23.38,39 Real-life data suggests however that anti-TNF therapies have a higher risk of serious infections,40 and that patients on ustekinumab are less likely to withdraw from treatment due to adverse events.41 Notwithstanding specific circumstances, anti-TNF therapies are being superseded in terms of both efficacy and safety by the newer drugs.
On drug survival, approximately 50% patients withdraw from anti-TNF therapy within 3 years; drug survival is significantly higher with ustekinumab than with adalimumab, infliximab, or etanercept.42 Whilst still a matter of debate, drug survival rates with IL-17 inhibitors probably lie between the range of ustekinumab and other anti-TNF responses; there is no conclusion yet on this aspect for guselkumab or the other IL-23 inhibitors, but research is ongoing.
In a patient with no comorbidities, ustekinumab, IL-17 inhibitors, and IL-23 inhibitors are all safe options, but comorbidities can drive the choice of drug (Table 1). For example, where a patient has severe psoriatic arthritis, robust data supports the use of the anti-TNF as the gold standard. The anti-IL-17 therapies would be second choice, and ustekinumab and the IL-23 inhibitors third. This does not mean that all patients with psoriatic arthritis should be treated with an anti-TNF; a patient with mild psoriatic arthritis and severe psoriasis could be appropriately treated with one of the other therapies.
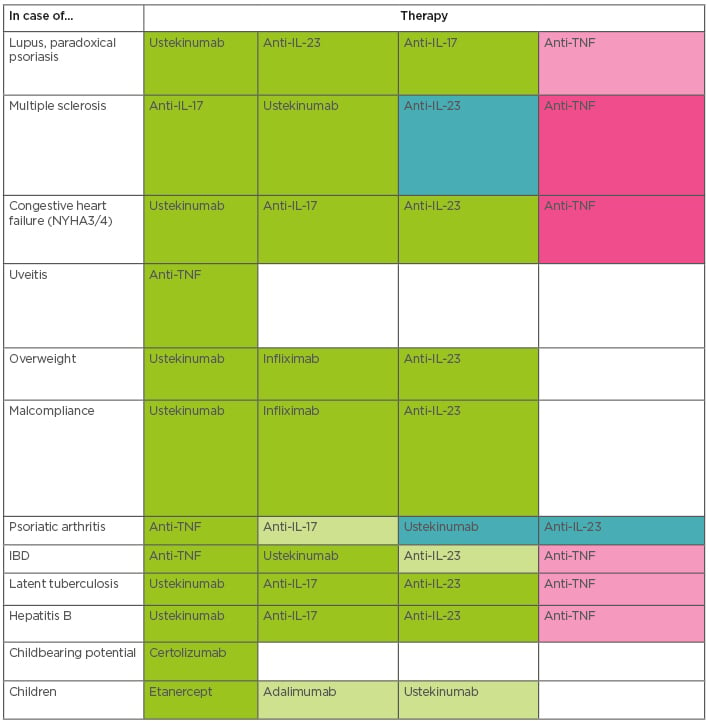
Table 1: Treatment choices in psoriasis with comorbidity.
A suggested scheme for treatment choice in psoriasis patients with comorbidities. The colours show the order of preference: dark green for the first-choice therapy, followed by paler green, then pink. Dark pink indicates that therapies would normally be avoided for patients with these comorbidities.
Anti-TNF: Anti-tumour necrosis factor; IBD: inflammatory bowel disease; IL: interleukin; NYHA: New York Heart Association.
The development of IL-17A inhibitor secukinumab as a treatment for IBD was stopped because of lack of efficacy and higher rates of adverse events compared to placebo;43 new onset IBD and aggravation of existing disease was reported. As described by Prof Reich, this led to the description of pathogenic and nonpathogenic Th17 cells44 and new understanding that suppression of IL-17 can lead to aggravation of IBD. For a patient with a family or personal history of IBD, the first choice of psoriasis therapy would be an anti-TNF or ustekinumab, which are indicated in Crohn’s disease. Studies on IL-23 inhibitors are still ongoing, but this class of drug is likely to have a beneficial effect on IBD as well. Etanercept is not effective in IBD but will not exacerbate the condition; IL-17 inhibitors should be used only with caution in these patients.
Risk of TB reactivation is a specific class effect of the anti-TNF therapies;45 therefore, ustekinumab, IL-17 inhibitors, and IL-23 inhibitors are all preferable to anti-TNF for a patient with latent TB. The necessity of TB screens might no longer be obligatory in the future when prescribing drugs other than anti-TNF. In a patient with acute infection with HBV, biologic treatment should be avoided before antiviral therapy is initiated. Where the infection is occult, reactivation can occur with ustekinumab or IL-17 inhibitors but only in patients who test positive for hepatitis B surface antigen or DNA-detected HBV.46,47 With that caveat, ustekinumab, anti-IL-17, and anti-IL-23 are safe choices in patients with a history of HBV infections, whereas HBV reactivation has been shown for anti-TNF treated patients.
Paradoxical psoriasis and lupus or lupus-like syndrome have been shown to be a class effect of anti-TNF drugs,48 and there is ongoing discussion on whether anti-TNF approaches increase the risk of demyelinating disorders and lymphoma.
Discontinuation of treatment during pregnancy is often the preferred approach; however, a study on pregnant women treated with certolizumab pegol found, at the time of birth, that women had expected levels of the drug in blood samples; the infants had minimal or no detectable levels.49 The study concluded there was a lack of placental transfer of certolizumab pegol during pregnancy; this drug would therefore be preferable to others in pregnant women if psoriasis treatment is necessary.
In European children, adalimumab4 has been indicated since 2015 for those of ≥4 years of age; etanercept2 since 2004 for those of ≥6 years of age; and ustekinumab5 since 2015 for those of ≥12 years of age. Etanercept is the first-choice therapy in children because of long experience, and also because etanercept can be stopped after initial treatment and restarted, which is particularly useful as the disease course can alter during adolescence.
For the future, research is ongoing into biomarkers, which will predict a patient’s response. HLA CW6 predicts better response to ustekinumab. Drug levels of adalimumab measured as early as Week 1 may predict response; the BSTOP Study Group/PSORT Consortium found that after a single injection of 80 mg adalimumab, blood levels ≥7 µg/mL suggests 80% probability of achieving PASI 75 response and 51% probability of achieving PASI 90 response.50 Blood levels <3 µg/mL indicate that treatment response will be low. While this is useful clinical information, Prof Conrad said the ideal is to find biomarkers to drive decisions prior to initiation of treatment.
Summarising this message, he said that there is not a single biologic agent or class which can be used to treat all patients. All classes have unique benefits and limitations depending on drug-related, patient-related, or disease-related factors.
Conclusion
Prof Reich concluded the symposium by stating that the introduction of IL-23 inhibitors has broadened the therapeutic armamentarium in psoriasis, to the extent that many patients on IL-23 inhibitors have a PASI 100 response after a year. The IL-23 inhibitors give high levels of response, seem to be extremely safe, and instill stable responses in patients. Great things have clearly been achieved in the treatment of psoriasis.
WATCH THE FULL SYMPOSIUM ONLINE
EM-17705







