Meeting Summary
Prof Reich welcomed delegates to the satellite symposium and explained that the aims of the meeting were to introduce the clinical role of targeted interleukin (IL)-23 therapies in psoriasis, show why IL-23 therapy is effective against psoriasis, show how it works in patients by illustrating emerging clinical trial data, and, finally, describe how the IL-23 inhibitors can be used to address unmet clinical needs in patients with psoriasis. Dr Blauvelt started the meeting by providing an update on the current understanding of the immunology of cytokine pathways in psoriasis. Prof Reich then gave an overview of the clinical value of IL-23 inhibitors as novel targeted treatments for psoriasis, summarising data from pivotal clinical trials that have been carried out to support the introduction of these treatments into the clinical armamentarium. Finally, Prof Girolomoni reviewed the indications for biologic therapies and discussed how IL-23 inhibitors can be integrated into the current therapeutic environment. The satellite symposium concluded with a lively question and answer session.
An Immunologic Understanding of Cytokine Pathways in Psoriasis
Doctor Andrew Blauvelt
Psoriasis has a highly complex pathophysiology driven by increased T helper (Th) cell activity resulting in inflammation, overproduction and activation of keratinocytes, and the formation of psoriasis plaques. IL-23 is a key upstream regulatory cytokine in psoriasis pathogenesis. Produced by antigen-presenting dendritic cells, the normal function of IL-23 is to stimulate differentiation, activation, proliferation, and survival of Th17 cells. Specialised Th17 cells are normally involved in the adaptive response utilised in mucocutaneous defence against infection by extracellular organisms such as Candida albicans or Staphylococcus aureus, which may also play a role in pathogenesis of psoriasis (Figure 1).1-4 IL-23 is composed of two molecular subunits, p19 and p40; blockade of IL-23 can be achieved by targeting either subunit, but only p19 subunit inhibition specifically blocks the IL-23 cytokine. Ustekinumab, a biologic therapy for psoriasis and psoriatic arthritis, is an inhibitor of p40, and results in the blockade of IL-12 as well as IL-23. The focus of current clinical research has been the specific inhibition of IL-23 via more targeted inhibition of the p19 subunit alone. In patients with psoriasis, overproduction of IL-23 occurs in the upper dermis, leading to excessive Th17 cell accumulation and overproduction of IL-17A and IL-22. This leads to keratinocyte proliferation and activation, pro-inflammatory cytokine production (e.g., tumour necrosis factor [TNF]-α), and neutrophil accumulation.
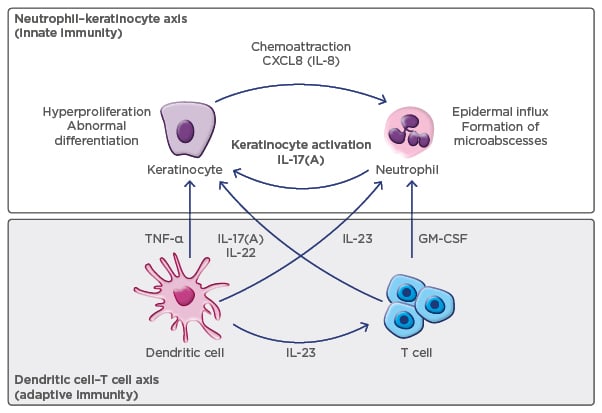
Figure 1: Model of psoriasis pathogenesis.2
GM-CSF: granulocyte-macrophage colony-stimulating factor; IL: interleukin; TNF: tumour necrosis factor.
Psoriasis is associated with genetic polymorphisms in the p19 and p40 subunit genes of IL-23, as well as in IL-23R, a gene that encodes for a subunit of the IL-23 receptor present on the cell surface of Th17 cells.5 A defect in IL-23R has been shown to be protective against the development of psoriasis by impairing IL-23-induced Th17 effector responses in humans.6 Importantly, IL-17A, produced by Th17 cells and other cell types, is a downstream effector cytokine in psoriasis pathogenesis. There is evidence from animal studies, as well as human tissue studies, that blockade of IL-17 prevents the development of IL-23-mediated epidermal thickening and psoriasis-like disease.7 In contrast, the inhibition of IL-23 provides upstream inhibition of pathologic processes.
Blocking different targets in the immunopathogenic pathways involved in psoriasis has varying effects. Inhibition of the pathologic process with a broad immunosuppressant drug, such as methotrexate, is associated with more safety concerns and is less effective than more targeted inhibition of key cytokines such as IL-23 and IL-17A. Similarly, the mechanism of action of targeted inhibition of cytokine pathways has implications for safety and dosing. For example, loss of IL-17A activity is associated with the development of chronic mucocutaneous candidiasis in both mice and humans. Although there are currently no supporting scientific studies, it has been hypothesised that IL-23 blockade does not block all downstream IL-17 production (i.e., some residual IL-17A production remains from non-Th17 cells in the skin and gut); therefore, this may explain why IL-23 blockade may not lead to candidiasis or inflammatory bowel disease. To date, clinical evidence from studies of IL-23 inhibitors has shown no increase in the incidence of serious infections, reactivation of tuberculosis infection, hepatitis B, candidiasis, or inflammatory bowel disease. Blocking upstream targets, such as IL-23, is also associated with a need for less frequent dosing, since clinical efficacy of IL-23 inhibitors in psoriasis persists longer than serum drug levels. It is possible that IL-23 inhibition may cause the death of Th17 cells, which are dependent on IL-23 for cell survival, and thus could lead to prolonged disease control. Such considerations are based upon the basic understanding of the IL-23/Th17 immunologic pathway but require detailed tissue studies in humans to confirm.
The Clinical Value of Interleukin-23 Inhibitors
Professor Kristian Reich
Several IL-23 inhibitors are in clinical development, including guselkumab. It is the first IL-23 inhibitor to be approved for the treatment of patients with moderate-to-severe plaque psoriasis in the USA and is in Phase II evaluation for use in psoriatic arthritis. Other IL-23 inhibitors in clinical development include tildrakizumab and risankizumab, which are in Phase III, and mirikizumab, in Phase II.
Clinical Evidence: Guselkumab
The efficacy and safety of guselkumab has been evaluated in two recently published pivotal randomised, double-blind, placebo and active-controlled Phase III trials: VOYAGE 18 and VOYAGE 2.9 In VOYAGE 1, guselkumab was compared with adalimumab and placebo over a 1-year active comparator period, followed by a 4-year follow-up.8 The study included 837 patients, of whom 174 were initially randomised to placebo, 329 to guselkumab, and 334 to adalimumab. Co-primary endpoints included the proportions of patients achieving an Investigator Global Assessment (IGA) score of cleared or minimal disease (IGA 0 or 1), and ≥90% improvement in Psoriasis Area Severity Index (PASI 90) at Week 16 in the guselkumab group compared with placebo. The baseline patient characteristics were those of a typical psoriasis population: mean BMI of 30, mean overall PASI of 22, mean dermatology quality of life (QoL) index of 14, and a long duration of disease (mean: 18 years). Compared with placebo, a significantly higher percentage of patients on guselkumab achieved an IGA 0 or 1 (85.1% versus 6.9%, respectively; p<0.001) and PASI 90 (73.3% versus 2.9%, respectively; p<0.001). The response to guselkumab was rapid and the proportion of patients achieving PASI 100 at Week 16 was significantly higher for guselkumab than placebo (p<0.001). Responses to guselkumab were also significantly better than to adalimumab in the proportion of patients achieving IGA 0 or 1, PASI 90, and PASI 100. High level clinical responses were sustained to Week 48 (Figure 2).8 Guselkumab was effective in improving the scalp and nail manifestations of psoriasis, although the improvements compared with adalimumab were attenuated.8 Unpublished long-term data show that responses to guselkumab were sustained for up to 2 years, demonstrating excellent longevity of the therapeutic response.
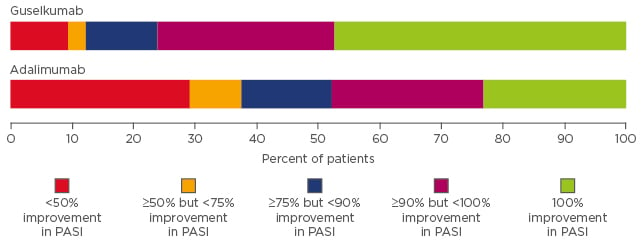
Figure 2: Distribution of psoriasis treatment responses (improvement in Psoriasis Area Severity Index) at Week 48 in the VOYAGE 1 trial.8
PASI: Psoriasis Area Severity Index.
A high level of treatment response has been shown to correlate with improved patient QoL. The Phase III clinical data from VOYAGE 1 show that the higher level of clinical efficacy in terms of PASI 90/100 response reported for guselkumab compared with adalimumab translates into significant and sustained improvements in QoL, as evidenced by higher Dermatology Life Quality Index (DLQI) scores.8
VOYAGE 2 had a similar design to VOYAGE 1, but included a period of randomised withdrawal (Weeks 24–28) followed by re-treatment or treatment switch (PASI 90 non-responders) through to Week 48.9 Co-primary endpoints were the same as in VOYAGE 1.
A total of 992 patients were randomised in a 2:1:1 ratio to guselkumab (496 patients), placebo (248 patients), and adalimumab (248 patients). Efficacy results were very similar to those of VOYAGE 1; clinical responses were observed early in the treatment period and, at Week 16, significantly higher proportions of patients achieved IGA 0 or 1, PASI 90, and PASI 100 compared with either placebo or adalimumab (p<0.001 for all comparisons with guselkumab).9 VOYAGE 2 also evaluated the effect of withdrawal of active treatment and demonstrated that the therapeutic efficacy of guselkumab was sustained after treatment was stopped. The mean time to loss of PASI 90 response was 15.0 weeks in the guselkumab-treated patients compared with 8.6 weeks in adalimumab-treated patients. In addition, 66% of patients who did not achieve a PASI 90 response to adalimumab achieved PASI 90 after switching to guselkumab at Week 28.9
The Phase III VOYAGE 1 and 2 safety data showed that guselkumab has a comparable safety profile to adalimumab with no new safety signals reported, resulting in a favourable risk:benefit profile. The incidence of overall infection, serious infections, and infections requiring antibiotic treatment were similar in guselkumab and adalimumab- treated patients.8,9
Clinical Experience with Other Interleukin-23 Inhibitors
Risankizumab is an IL-23 inhibitor that is in Phase II/III of clinical development. Data from a comparative clinical trial of risankizumab versus ustekinumab showed that in patients treated with risankizumab (dosed at Weeks 0, 4, and 16), 50% of patients maintained a PASI 90 response at Week 48; i.e., 32 weeks after the last risankizumab dose.10 These data provide further evidence of the sustainable effect of IL-23 inhibition in psoriasis, as seen in VOYAGE 2 with guselkumab. The immunological impact of targeted upstream IL-23 inhibition in the immunopathology of psoriasis requires further study to better understand this effect on the underlying disease process.
Another IL-23 in Phase III development is tildrakizumab. Data from the placebo-controlled reSURFACE 1 Part 1 trial11 show that although a significantly higher percentage of patients treated with tildrakizumab achieve PASI 75, PASI 90, and PASI 100 compared with placebo, the proportions of patients with PASI 90 and PASI 100 responses were lower than those reported for guselkumab or risankizumab.8-11 However, the proportion of patients achieving PASI 90 and PASI 100 improved at 28 weeks,11 suggesting that the time to treatment response may be longer with tildrakizumab; head-to-head comparisons are needed to better understand the efficacy of tildrakizumab.
IL-23 inhibitors also represent a promising new treatment option for patients with psoriatic arthritis. Guselkumab is the first anti-IL-23 biologic to demonstrate efficacy in psoriatic arthritis. Clinically significant effects on American College of Rheumatology (ACR) 20, ACR50, and ACR70 scores, enthesitis, and dactylitis at 24 weeks have been reported in a Phase II trial.12 In summary, in patients with moderate or severe psoriasis, IL-23 inhibitors are associated with high levels of clinical response, stable long-term responses that extend beyond serum drug levels, convenient injection intervals, and no safety concerns to date compared with other biologic treatments.
The Current Landscape of Psoriasis Treatments: When and Where to Embed Emerging Therapeutic Options
Professor Giampiero Girolomoni
Despite the introduction of new biologic treatments, there are a number of unmet needs in the clinical management of moderate-to-severe psoriasis, including late or inadequate use of systemic treatment, poor tolerability or effectiveness of conventional therapy in many patients, and effective treatment of psoriasis in difficult areas (scalp, genitalia, and palmoplantar areas).13,14 In addition, many current therapies (including biologics) lose optimal efficacy over time in a substantial proportion of patients. Severe psoriasis has a very significant impact on QoL, affecting the emotional,15 socio-familial,16 financial,17 work,18 and leisure19 aspects of patients’ daily lives. The systemic inflammation associated with severe psoriasis also puts patients at increased risk of metabolic disorders, such as Type 2 diabetes mellitus, non-alcoholic fatty liver disease, hypertension, and, ultimately, atherosclerosis and cardiovascular disease.20
Appropriate use of systemic therapy is very important, and treatment success requires the complete, or almost complete, clearance of psoriasis. Systemic therapy is indicated for patients with a PASI ≥10 or those with a PASI <10 who have involvement of the hands, scalp, face, nails, or palmoplantar or genital areas.21 Other indications include a body surface area (BSA) involvement of ≥5%, either where there is resistance to topical therapy or where patients are reluctant to use it; a BSA <5% with disseminated lesions; a patient’s subjective perception of disease severity (e.g., DLQI ≥10); active psoriatic arthritis; and psoriasis associated with severe symptoms (e.g., itch or burning) that are not controlled by topical therapies. Treatment goals should be agreed with patients after an informed discussion and re-evaluated after 3–4 months during treatment initiation and every 3–6 months during maintenance. The treatment efficacy goal that best correlates with disease remission and good patient satisfaction is an improvement in BSA of ≥90% (PASI 90); the targets for the maintenance phase are a minimum PASI of <1 or a BSA <1%, and a DLQI of <5.21,22 If treatment goals are not met, therapy may be changed or another drug may be added to the treatment regimen. A survey of the use of biologic therapy recently reported that many physicians also adjust either the dose or dose interval as a strategy to improve treatment response or maintain remission, even though this is an off-label approach and cannot be recommended.23 Important factors to be considered when selecting a systemic psoriasis treatment include age, body weight, treatment availability, disease severity, comorbidities, and concomitant diseases (Box 1).21,22
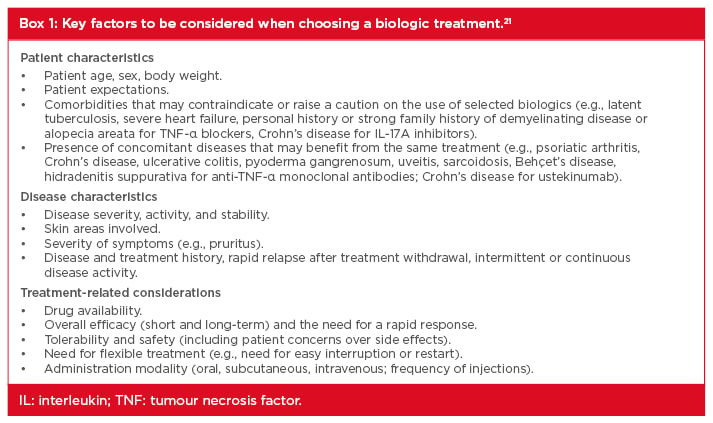
Box 1: Key factors to be considered when choosing a biologic treatment.21
IL: interleukin; TNF: tumour necrosis factor.
There is limited evidence to indicate which factors, if any, influence treatment outcomes. Age and body weight can have an impact on treatment efficacy, as well as disease severity and disease manifestations such as psoriatic arthritis. A multicentre study reported that patients who were genotyped positive for HLA-C*6 (generally younger patients) had a faster and greater response to treatment with the IL-23/IL-12 inhibitor ustekinumab.24 A French study25 recently reported that patients were more likely to be prescribed adalimumab than either etanercept or ustekinumab if they had severe psoriasis or if they had psoriatic arthritis. Younger patients (<30 years of age) and those who had positive screening for latent tuberculosis were more likely to receive ustekinumab than adalimumab. Patients with chronic obstructive pulmonary disease were also more likely to receive ustekinumab or etanercept than adalimumab, and there was a trend toward increased etanercept use in patients with cardiovascular comorbidities, metabolic syndrome, or a history of cancer. Systemic psoriasis treatments have distinct efficacy and safety profiles. Conventional systemic treatments such as methotrexate, cyclosporine, and dimethyl fumarate are associated with significant metabolic toxicity resulting in side effects (e.g., nausea, fatigue, headache, diarrhoea) and poor tolerability. TNF-α inhibitors have demonstrated greater tolerability compared with conventional therapy and are associated with longer drug survival times.26 Ustekinumab has also been reported to have higher drug persistence rates and longer drug survival than the TNF-α inhibitors etanercept, infliximab, and adalimumab.27
To conclude, the choice of treatment for a patient with moderate-to-severe psoriasis should involve a holistic decision-making approach, encompassing disease, patient, and treatment characteristics.
Question and Answer Session
Q: Why has candidiasis been noted in patients treated with IL-17 inhibitors but not in the clinical trials with IL-23 inhibitors?
A: Dr Blauvelt replied that an IL-17 inhibitor blocks all production of IL-17 from all cell types (Th17, neutrophils, innate lymphoid cells), and therefore, as IL-17 has a defensive role in the skin and gut, elimination of IL-17 would be expected to result in skin infections or gut inflammation. With IL-23 inhibition, a large proportion of IL-17 production will be removed, but a small amount (˜10%) of IL-17 production is not under IL-23 control, and it is hypothesised that this residual IL-17 is sufficient to protect the skin from Candida infection and the gut mucosa from inflammation.
Q: If you have a patient who is treated with adalimumab and does not achieve a PASI 90 response, what is the best treatment strategy?
A: Dr Blauvelt replied that if a patient is clearly not responding to treatment, the drug needs to be switched. In a patient with inadequate response, however, the situation is more difficult, and you can consider either switching or adding another drug to the regimen, such as methotrexate. Prof Reich added that dose adjustment is also an option; with adalimumab the normal dose is administered every 2 weeks but can be changed to weekly dosing on label, although this will double the cost of treatment.
Q: Can achieving and maintaining remission in psoriasis impact patients’ risk of cardiovascular disease?
A: Dr Blauvelt replied that there is an almost linear correlation between the level of systemic inflammation and the severity of psoriasis, and a patient with severe psoriasis is likely to have an increased risk of atherosclerosis and cardiovascular disease. Therefore, clearing psoriasis should improve cardiovascular risk by reducing inflammation. Some evidence is emerging to support this in the case of TNF-α inhibitors, but studies need to be carried out for IL-17 and IL-23 inhibitors.
Prof Reich added that, because atherosclerosis is an inflammatory process, treatment with an anti-inflammatory agent could reduce cardiovascular risk. If a psoriasis treatment could block pro-inflammatory cytokines in the heart vessels in addition to reducing the skin inflammation, it would have an impact on cardiovascular risk. The picture is not yet clear, but data are emerging showing that IL-17 inhibition may have positive effects on markers of cardiovascular risk.
Q: Why are we seeing differences in clinical responses with guselkumab, tildrakizumab, and risankizumab when they all target the same key cytokine, IL-23?
A: Prof Reich replied that there are also reported differences in the response to different TNF-α inhibitors. Blocking the same target does not mean the clinical response will be exactly the same; there will be differences in affinity, immunogenicity, and other aspects. Dr Blauvelt added that the mechanism of action is not the only consideration for treatment response; the drug must be dosed at the correct level and at the right frequency, because these factors also influence efficacy.
Q: Do you think that treatment with guselkumab is disease-modifying?
A: Prof Reich replied that, at present, only very preliminary observations can be made in this regard. IL-23 inhibitors, as a class, have a clear sustained efficacy that persists months beyond their pharmacokinetics and provides a lasting clinical response for a substantial subgroup of patients. More data from biopsy studies are required before this can be described as disease modification, but it seems likely that research is taking us closer to disease modification in the future. Prof Girolomoni and Dr Blauvelt agreed with Prof Reich’s views.







