Presenters: Richard B. Warren,¹ Andrew Blauvelt,² Jerry Bagel,3 Abel Jarell,4 Kristian Reich,5 Kim Papp,6 Adelaide Hebert7
1. Dermatology Centre, Manchester NIHR Biomedical Research Centre, The University of Manchester, UK
2. Oregon Medical Research Center, Portland, Oregon, USA
3. Psoriasis Treatment Center of Central New Jersey, East Windsor, New Jersey, USA
4. Northeast Dermatology Associates, Portsmouth, New Hampshire, USA
5. Translational Research in Inflammatory Skin Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
6. K. Papp Clinical Research and Probity Medical Research, Waterloo, Canada
7. University of Texas McGovern Medical School, Houston, Texas, USA
Disclosure: Warren has received consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, and UCB Pharma; research grants from AbbVie, Almirall, Janssen, LEO Pharma, Novartis, and UCB Pharma. Blauvelt reported being a scientific adviser for AbbVie, Aligos, Almirall, Amgen, Arcutis, Arena, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Evommune, Forte, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Rapt, Regeneron, Sanofi Genzyme, Sun Pharma, and UCB Pharma; and has been a clinical study investigator for AbbVie, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron, Sun Pharma, and UCB Pharma. Bagel has received research funding payable to Psoriasis Treatment Center from AbbVie, Amgen, Arcutis Biotherapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Corrona, LLC, Dermavant Sciences, Ltd., Dermira, UCB, Eli Lilly and Company, Glenmark Pharmaceuticals Ltd., Janssen Biotech, Kadmon Corporation, LEO Pharma, Lycera Corp, Menlo Therapeutics, Novartis, Pfizer, Regeneron Pharmaceuticals, Sun Pharma, Taro Pharmaceutical Industries Ltd., and Ortho Dermatologics; consultant fees from AbbVie, Amgen, Celgene Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Janssen Biotech, Novartis, Sun Pharmaceutical Industries Ltd., and UCB; and speaker fees from AbbVie, Celgene Corporation, Eli Lilly, Janssen Biotech, and Novartis. Jarell has served as a scientific advisor or clinical study investigator for AbbVie, Asana BioSciences, Bristol Myers Squibb, Castle Biosciences, Celgene, Dermira, Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, LEO Pharma, Novartis, Pfizer, Purdue Pharma, Regeneron, Sanofi Genzyme, Sienna Biologics, Sun Pharma, and UCB Pharma; and has served as a paid speaker for Castle Biosciences, Eli Lilly and Company, Novartis, Regeneron, and Sanofi Genzyme. Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Amgen, Biogen, Boehringer Ingelheim Pharma, Bristol Myers Squibb, Celgene, Centocor, Covagen, Eli Lilly, Forward Pharma, Galderma, GlaxoSmithKline, Janssen-Cilag, LEO, Medac, Merck Sharp & Dohme Corp., Novartis, Ocean Pharma, Pfizer, Regeneron, Sanofi, Takeda, UCB Pharma, and Xenoport. Papp is an advisor and investigator for Novartis. Hebert has received honoraria from Biofrontera, Cassiopea, Galderma, LEO, Ortho Dermatologics, and Pfizer; served on data and safety monitoring boards for Bausch Health, GlaxoSmithKline, and Regeneron; and received research grants (paid to UTHealth McGovern Medical School) from Galderma, LEO, Mayne, Ortho Dermatologics, Pfizer, Promius, Sienna, and UCB.
Acknowledgements: Medical writing assistance was provided by Jennifer Taylor, London, UK. Our thanks are given to the presenters and authors of the ePosters summarised in this article.
Support: This article was funded by an educational grant from UCB Pharma, who had no input into its content. The views and opinions expressed are those of the speakers.
Citation: EMJ Dermatol. 2021;9[Suppl 7]:2-10.
Meeting Summary
Richard B. Warren highlighted Psoriasis Symptoms and Impacts Measure (P-SIM) responses from the Phase III BE VIVID trial in patients receiving the IL-17A/F inhibitor bimekizumab (BZK) versus the IL-12/23 inhibitor ustekinumab (UST) or placebo (PBO). Warren also presented P-SIM responses, focusing on the BE SURE trial in patients receiving BZK versus the TNF inhibitor adalimumab (ADA). In a further presentation from the BE SURE trial, Andrew Blauvelt discussed how improved disease control translates into quality of life (QoL) in patients receiving BZK compared with ADA. Jerry Bagel highlighted patient-reported outcomes (PROs) following treatment with the IL-17A inhibitor ixekizumab (IXE), the TNF inhibitor etanercept (ETN), and UST from the UNCOVER-3 and IXORA-S Phase III trials. Additional findings with IXE were discussed by Abel Jarell in an oral presentation on the durability of skin clearance improvement with IXE versus the IL-23 inhibitor guselkumab (GUS) in the IXORA-R trial. Further GUS results were presented from the Phase III VOYAGE 2 trial on health-related quality of life (HRQoL), depression and anxiety, and work productivity up to 5 years. In another analysis with long-term follow-up, Kim Papp reported effectiveness and QoL outcomes in patients treated with the IL-17A inhibitor secukinumab (SEC) for up to 36 months in the international PURE registry. Moving to the IL-17 receptor A inhibitor brodalumab (BRO), Adelaide Hebert presented a post hoc analysis of Psoriasis Symptom Inventory (PSI) response rates and scores stratified by sex and skin clearance levels in the AMAGINE-2 and AMAGINE-3 trials.
Psoriasis Symptoms and Impacts Measure (P-SIM) Responses: The BE VIVID Bimekizumab in Moderate-to-Severe Plaque Psoriasis Phase III Trial
Richard B. Warren
Warren presented P-SIM responses from the Phase III BE VIVID trial in patients with moderate-to-severe plaque psoriasis.1,2 P-SIM is a novel, validated PRO tool developed to capture key signs, symptoms, and life impacts of plaque psoriasis.3
P-SIM data were used to compare patient-perceived itching, pain, and scaling in patients receiving the IL-17A/F inhibitor BZK versus the IL-12/23 inhibitor UST or PBO. P-SIM items were scored daily (0–10; no–very severe) using a handheld PRO device and averaged weekly through Week 16. The authors reported the proportions of patients achieving a ≥4-point reduction from baseline (indicating marked clinically meaningful improvement) and a score of 0 or 1 (no or minimal impact of the symptom).
A total of 567 patients were randomly allocated in a 4:2:1 ratio to BZK 320 mg every 4 weeks (q4w; n=321); UST 45/90 mg (dosing was based on body weight) at baseline and Week 4 then every 12 weeks (n=163); or PBO (n=83) to Week 16 then BZK q4w to Week 52.
The authors reported that at Week 16, the proportions of patients receiving BZK, UST, and PBO achieving a ≥4-point reduction from baseline were 68.0%, 54.8%, and 11.3% for itching (BZK versus PBO: p<0.001; BZK versus UST: nominal p<0.02); 73.7%, 60.0%, and 10.4% for pain (BZK versus PBO: p<0.001; BZK versus UST: nominal p<0.02); and 76.0%, 56.7%, and 10.7% for scaling (BZK versus PBO: p<0.001; BZK versus UST: nominal p<0.001), respectively (Figure 1).
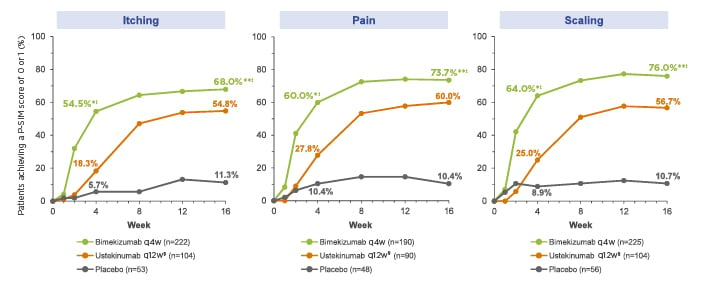
Figure 1: Proportions of patients achieving a ≥4-point reduction from baseline in P-SIM Score (NRI).
Patients included in the analysis had a baseline score 4 for the P SIM item of interest.
Missing data were imputed using NRI; if scores were missing for >3 days in a week, the weekly score was considered missing. Comparisons between treatment groups were based on the Cochran Mantel Haenszel test for the general association.
*nominal p<0.001 versus placebo.
†nominal p<0.02 versus ustekinumab.
‡nominal p<0.001 versus ustekinumab.
§Ustekinumab dosing was based on weight: patients ≤100 kg at baseline received one ustekinumab 45 mg injection and one placebo injection; patients >100 kg at baseline received two ustekinumab 45 mg injections.
NRI: non-responder imputation; P-SIM: Psoriasis Symptoms and Impacts Measure; q4w: every 4 weeks; q12: every 12 weeks.
The proportions of patients receiving BZK, UST, and PBO achieving a P-SIM score of 0 or 1 at Week 16 were 51.6%, 38.0%, and 3.1% for itching (BZK versus PBO: nominal p<0.001; BZK versus UST: nominal p<0.02); 63.8%, 43.6%, and 7.0% for pain (BZK versus PBO: nominal p<0.001; BZK versus UST: nominal p<0.001); and 68.3%, 37.1%, and 3.0% for scaling (BZK versus PBO: nominal p<0.001; BZK versus UST: nominal p<0.001), respectively.
The authors noted that greater proportions of BZK-treated patients reported rapid and marked clinically meaningful improvements in itching, pain, and scaling after only one dose compared to UST and PBO.
Psoriasis Symptoms and Impacts Measure (P-SIM) Responses: The BE SURE Bimekizumab in Moderate-to-Severe Plaque Psoriasis Phase III Trial
Richard B. Warren
Warren presented P-SIM responses from the BE SURE trial.4 Patient-perceived itching, pain, and scaling were compared for patients receiving BZK versus the TNF inhibitor ADA through Week 24.
A total of 478 patients were randomised 1:1:1 to BZK 320 mg q4w (n=158); BZK q4w to Week 16 then every 8 weeks (q8w) to Week 56 (n=161); or ADA per label to Week 24 then BZK q4w to Week 56 (n=159).
Regarding early PROs at Week 4, the proportions of patients receiving BZK q4w, BZK q4w/q8w, and ADA achieving a ≥4-point reduction from baseline were 49.2%, 50.0%, and 20.6% for itching; 51.4%, 58.3%, and 20.7% for pain; and 56.6%, 58.9%, and 18.3% for scaling, respectively (BZK versus ADA: nominal p≤0.001 for all comparisons). In the longer-term, at Week 24, the corresponding proportions were 66.7%, 65.6%, and 45.8% for itching (BZK versus ADA: nominal p≤0.004); 70.1% (versus ADA: nominal p≤0.001), 67.8% (versus ADA: nominal p≤0.004), and 46.7% for pain; and 69.7% (versus ADA: nominal p≤0.001), 65.9% (versus ADA: nominal p≤0.004), and 47.7% for scaling, respectively.
The authors also showed the proportions of patients receiving BZK q4w, BZK q4w/q8w, and ADA that achieved a P-SIM Score of 0 or 1. For itching, these were 21.5%, 24.8%, and 6.6% at Week 4 (BZK versus ADA: nominal p<0.001); and 50.0%, 50.4% (versus ADA: nominal p<0.05), and 38.0% at Week 24, respectively. For pain, the corresponding figures were 34.4%, 43.3%, and 10.7% at Week 4 (BZK versus ADA: nominal p<0.001); and 56.6%, 58.2% (versus ADA: nominal p<0.05), and 45.5% at Week 24, respectively. For scaling, the results were 31.3%, 34.5%, and 7.4% at Week 4 (BZK versus ADA: nominal p<0.001); and 54.7% (versus ADA: nominal p=0.007), 57.6% (versus ADA: nominal p<0.001), and 37.7% at Week 24, respectively.
The authors concluded that greater proportions of patients treated with BZK reported rapid and clinically meaningful improvements in itching, pain, and scaling and either no or minimal symptoms compared with ADA by Week 24.
Bimekizumab Versus Adalimumab in Plaque Psoriasis: Higher Efficacy Translates into Improvements in Quality of Life in the BE SURE Trial
Andrew Blauvelt
In a further presentation from the BE SURE trial,5 Blauvelt discussed the relationship between treatment efficacy and QoL in patients with moderate-to-severe plaque psoriasis receiving BZK or ADA.
QoL was assessed using the Dermatology Life Quality Index (DLQI), with DLQI 0/1 indicating no impact of psoriasis on QoL. For psoriasis severity, complete skin clearance was indicated by Psoriasis Area and Severity Index (PASI)=0 wand body surface area (BSA)=0%, while disease control was indicated by PASI ≤2 and BSA ≤1%.
The authors reported data at Weeks 24 and 56 for patients independently and simultaneously achieving absolute PASI or BSA thresholds and DLQI 0/1 in the BZK q4w, BZK q4w/q8w, and ADA groups.
At Week 24, higher proportions of patients treated with BZK q4w and q4w/q8w achieved complete skin clearance, disease control, and no impact on QoL compared to ADA.
For complete skin clearance, proportions achieving PASI=0 at Week 24 in the BZK q4w, BZK q4w/q8w, and ADA groups were 71.8%, 71.1%, and 32.0%, respectively. For BSA=0%, the corresponding proportions were 71.8%, 71.8%, and 32.7%, respectively. For disease control at Week 24, the proportions of patients achieving PASI ≤2 in the BZK q4w, BZK q4w/q8w, and ADA groups were 91.3%, 94.0%, and 59.9%, respectively. In addition, for BSA ≤1%, the corresponding proportions were 83.2%, 83.2%, and 45.6%, respectively.
Regarding QoL at Week 24, DLQI 0/1 was achieved by 71.1%, 73.5%, and 52.4% in the BZK q4w, BZK q4w/q8w, and ADA groups, respectively.
Considering the psoriasis severity and QoL measures together at Week 24, greater proportions of patients treated with BZK q4w and q4w/q8w simultaneously achieved DLQI 0/1 and PASI or BSA thresholds compared to ADA. Both PASI=0 and DLQI 0/1 were achieved by 55.4%, 55.8%, and 24.8% in the BZK q4w, BZK q4w/q8w, and ADA groups, respectively. The corresponding proportions for both BSA=0 and DLQI 0/1 were 55.4%, 56.5%, and 24.8%, respectively.
At Week 56, the majority of patients in both BZK groups achieved complete skin clearance, as demonstrated by PASI=0 for 81.4% and 79.0% in the q4w and q4w/q8w groups, respectively. Simultaneous achievement of both PASI=0 and DLQI 0/1 occurred in 77.9% and 76.8% of the q4w and q4w/q8w groups, respectively.
The authors concluded that at Week 24, patients treated with BZK showed substantially higher levels of skin clearance with no impact of psoriasis on QoL, relative to patients treated with ADA. After 1 year of treatment with BZK, >75% of patients achieved complete skin clearance and reported no residual impact of psoriasis on QoL.
Sustained and Rapid Improvements in Patient-Reported Outcomes for Patients with Moderate-to-Severe Psoriasis with Moderate and High Body Surface Area Treated with Ixekizumab: UNCOVER-3 and IXORA-S
Jerry Bagel
Bagel highlighted PROs following treatment with the IL-17A inhibitor IXE, the TNF inhibitor ETN, and UST in adults with moderate-to-severe plaque psoriasis. Side-by-side results were presented from two Phase III trials.6
UNCOVER-3 compared the efficacy and safety of IXE to ETN and PBO. Patients received PBO, ETN, or IXE (80 mg q4w or every 2 weeks [q2w]). Results were reported for patients who completed 12 weeks of PBO, ETN or IXE q2w, followed by IXE q4w through Week 264 in the extension period. IXORA-S compared the efficacy and safety of IXE with UST. Results were reported for patients who received IXE or UST label dosing through 52 weeks.
The authors reported response rates and median times to first itch Numeric Rating Scale (NRS)=0 and Patient Global Assessment (PatGA)=0. Treatment groups were stratified by baseline, middle, or upper BSA 10–20% or >20%.
Regarding response rates in UNCOVER-3 at Week 264, the proportions of patients with BSA 10–20% achieving itch NRS=0 in the PBO, ETN, and IXE q2w/q4w groups were 50.8%, 54.8%, and 54.1%, respectively. The corresponding proportions for patients with BSA >20% were 52.0%, 50.3%, and 46.9%, respectively. Looking at PatGA=0 in the subgroup of patients with BSA 10–20%, this indicator was achieved by 50.0%, 56.1%, and 57.5% of the PBO, ETN, and IXE q2w/q4w groups, respectively. The corresponding rates for the BSA >20% subgroup were 48.1%, 50.7%, and 46.9%, respectively.
Response rates at Week 52 in the BSA 10–20% subgroup of IXORA-S were higher for IXE q2w/q4w compared to UST for both itch NRS=0 (50.9% versus 33.3%) and PatGA=0 (52.8% versus 36.1%). In the BSA >20% subgroup, itch NRS=0 rates were similar (IXE: 36.9%; UST: 37.0%) while response rates for PatGA=0 were higher in the IXE groups (45.6%) compared to the UST group (32.9%).
Median times were reported next. For the BSA 10–20% subgroup, median weeks to itch NRS=0 in UNCOVER-3 were 12.4, 25.1, and 24.0 in the IXE, ETN, and PBO groups, respectively. In IXORA-S, median times were 12.1 and 28.7 weeks for IXE and UST, respectively. For BSA >20%, median weeks to itch NRS=0 in UNCOVER-3 were 12.1, 24.6, and 24.1 in the IXE, ETN, and PBO groups, respectively. In IXORA-S, median times were 16.3 and 24.0 weeks for IXE and UST, respectively. The authors noted that similar trends in median time to first PatGA=0 response were observed.
They concluded that patients with baseline BSA 10–20% or >20% treated with IXE demonstrated sustained high levels of complete resolution of itch and elimination of disease severity through 264 weeks. Median times to itch NRS=0 and PatGA=0 were shorter for IXE than for ETN or UST in both BSA subgroups.
Ixekizumab Provides More Durable Improvements in Skin Clearance, Itch, and Quality of Life than Guselkumab in Patients with Moderate-to-Severe Psoriasis: 24-week Results From IXORA-R
Abel Jarell
Jarell gave an oral presentation of an ePoster with results on skin clearance, itch, and QoL in patients with moderate-to-severe psoriasis treated with IXE versus GUS in the IXORA-R trial.7
IXORA-R was a Phase IV, randomised, double-blind, head-to-head, multicentre trial comparing the efficacy and safety of IXE with GUS. It was previously reported that significantly more patients treated with IXE versus GUS achieved the primary endpoint of complete skin clearance (PASI 100) at Week 12.8
In the current analysis, the authors compared the durability of skin clearance improvement (PASI 90 and PASI 100) with IXE versus GUS over 24 weeks of treatment. In addition, the authors evaluated the associations of durable improvement in PASI 90 and PASI 100 with QoL (DLQI 0/1) and itch NRS=0 at 24 weeks.
A total of 1,027 patients were randomised 1:1 to standard dosing of IXE (n=520) or GUS (n=507) for 24 weeks. Regarding the durability of skin clearance improvement, a greater proportion of patients treated with IXE maintained PASI 90 for >12 consecutive weeks compared to those receiving GUS (64% versus 47%, respectively; p<0.001). The corresponding proportions maintaining PASI 100 for >12 consecutive weeks were 32% and 19%, respectively (p<0.001).
Jarell presented the DLQI 0/1 and itch NRS=0 response rates at Week 24 stratified by >12 versus <12 consecutive weeks with PASI 90 or PASI 100. Patients who maintained PASI 90 or PASI 100 for ≥12 versus <12 consecutive weeks were more likely to achieve DLQI 0/1 (PASI 90: 80% versus 56%; PASI 100: 84% versus 65%; p<0.001) and itch NRS=0 (PASI 90: 59% versus 31%; PASI 100: 72% versus 39%; p<0.001) at Week 24.
Jarell concluded that IXE was superior to GUS in providing durable skin clearance as measured by PASI 90 or PASI 100. In addition, patients with longer duration of skin clearance were more likely to have complete itch resolution and psoriasis was less likely to impact QoL.
Health-Related Quality of Life and Work Productivity in Patients with Moderate-to-Severe Psoriasis Treated with Guselkumab: 5-year Data from VOYAGE 2
Kristian Reich
Results from the Phase III VOYAGE 2 trial9 previously demonstrated greater improvements in measures of HRQoL, depression and anxiety, and work productivity in patients with moderate-to-severe plaque psoriasis treated with GUS compared to PBO at Week 16 and ADA at Week 24.10 In this analysis, Kristian Reich presented results from Week 100 through Week 252 (5 years).9
A total of 992 patients were randomly allocated to either GUS 100 mg at Weeks 0, 4, and 12 and then q8w; PBO at Weeks 0, 4, and 12, followed by GUS 100 mg at Weeks 16 and 20, and then q8w; or ADA 80 mg at Week 0, 40 mg at Week 1, and finally 40 mg q2w until Week 23. Randomised withdrawal was from Weeks 28–72 and open-label GUS 100 mg q8w from Weeks 76–252.
HRQoL was assessed using the 36-item Short-Form (SF-36), from which the Physical Component Score (PCS) and Mental Component Score (MCS) are derived. An improvement of ≥5 points in PCS and MCS is considered clinically meaningful. The Hospital Anxiety and Depression Scale (HADS) subscales for anxiety (HADS-A) and depression (HADS-D) were used, with scores ≥8 indicating anxiety or depression, respectively. The Work Limitations Questionnaire (WLQ) was used to determine on-the-job disability in time-management, physical, mental-interpersonal, and output scores.
The combined GUS group was defined as patients randomised to GUS at baseline, those randomised to PBO at baseline who crossed over to GUS at Week 16, and patients randomised to ADA at baseline who crossed over to GUS at or after Week 28. The authors reported that at Week 100 (n=853), 46.7% and 45.8% achieved clinically meaningful improvement in SF-36 PCS and MCS, respectively, and the results were maintained through Week 252 (n=727; 44.6% and 48.7%).
At Week 100, 55.9% of patients with baseline HADS-A ≥8 (n=329) reported HADS-A <8 and 56.8% of patients with baseline HADS-D ≥8 (n=236) reported HADS-D <8. The results were maintained through Week 252 (HADS-A <8: 60.1% [n=276]; HADS-D <8: 57.4% [n=195]).
The authors reported mean reductions from baseline in the four WLQ domains at Week 100, which were maintained through Week 252. They concluded that in patients with moderate-to-severe psoriasis, sustained improvements were observed in general HRQoL, depression and anxiety, and work productivity through up to 5 years of GUS treatment.
Secukinumab Helps Achieve Clear or Almost Clear Skin and Improve Quality of Life in Psoriasis over 36 Months of Treatment: The PURE Study
Kim Papp
Papp reported effectiveness and QoL outcomes in patients with moderate-to-severe plaque psoriasis treated with the IL-17A inhibitor SEC for up to 36 months in the international PURE registry.11
The registry enrolled 2,362 adult patients in a 1:1 ratio of SEC to other treatments, of whom 89.1% were from Canada. Papp reported the findings in patients treated with SEC, including baseline characteristics and 3-year results for mean PASI score, clear or almost clear skin (Investigator Global Assessment [IGA] 0/1), and QoL (DLQI 0/1).
As of January 2020, 848 patients were receiving SEC. Regarding disease severity according to IGA, the authors observed that IGA 0/1 response was reported by 1.8% patients at baseline (n=841), 65.6% at 18 months (n=328), and 61.4% at 36 months (n=75). The mean PASI score was 13.3 at baseline (n=840), 1.8 at 18 months (n=326), and 1.6 at 36 months (n=73). Regarding QoL, DLQI 0/1 response was reported in 3.1% at baseline (n=829), 56.0% at 18 months (n=259), and 61.7% at 36 months (n=47) (Figure 2).
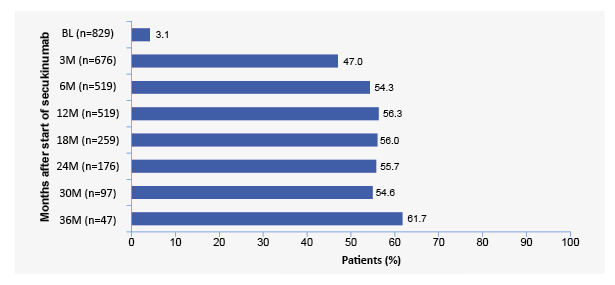
Figure 2: Percentage of secukinumab-treated patients reporting DLQI 0/1 response up to 36 months.
BL: baseline; DLQI: Dermatology Life Quality Index; M: months; n: number of patients.
The authors concluded that patients treated with SEC demonstrated sustained improvement in PASI scores, which was mirrored by the proportion with clear or almost clear skin up to 36 months, as well as minimal or no impact of psoriasis on quality of life.
Patient-Reported Psoriasis Symptoms Stratified by Sex and Level of Skin Clearance in Clinical Trials of Brodalumab
Adelaide Hebert
Hebert presented a post hoc analysis of PSI response rates and scores stratified by sex and skin clearance levels in clinical trials of the IL-17 receptor A inhibitor BRO.12 The PSI is a validated, 8-item tool measuring patient-reported psoriasis signs and symptoms, with total scores ranging 0–32 and higher scores indicating increased severity.13 The PSI contains items on itching, redness, scaling, burning, stinging, cracking, flaking, and pain.
Data were analysed from two Phase III studies (AMAGINE-2 and AMAGINE-3)14 in patients with moderate-to-severe psoriasis who received continuous BRO 210 mg q2w or UST through 12 weeks. Rates of PSI response (total score ≤8 and no item scores >1) were stratified by sex. Mean PSI scores were stratified by sex and PASI score (75–<90, 90–<100, and 100).
Regardless of sex, BRO 210 mg q2w was associated with significantly higher PSI response rates compared to UST from Week 2 through Week 12. At Week 12, PSI response rates with BRO and UST, respectively, were 72.3% versus 59.7% in males and 67.3% versus 57.5% in females.
Results for the three PASI groups are shown in Figure 3. Among patients with PASI 75–<90 at Week 12, BRO was associated with numerically lower mean PSI scores compared to UST for both males (4.89 versus 5.85) and females (6.40 versus 7.37).
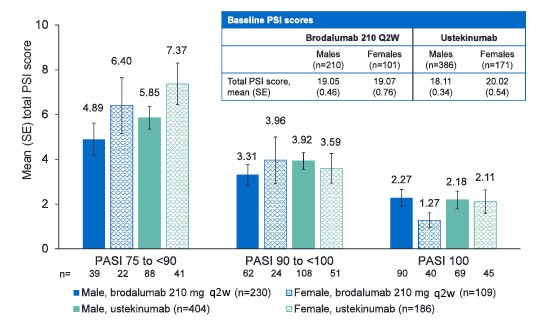
Figure 3: Mean total PSI scores at Week 12 stratified by sex and PASI scores.
PASI: Psoriasis Area and Severity Index; PSI: Psoriasis Symptom Inventory; q2w: every 2 weeks; SE: standard error.
The authors concluded that in male and female patients BRO was associated with achievement of PSI response by Week 2, with a trend toward greater symptom relief for those with incomplete PASI responses at Week 12 compared to UST.