Meeting Summary
Substantial developments over the last decade have advanced our knowledge and understanding of psoriasis, from both a basic scientific and clinical perspective. Identification of immunopathological mechanisms involved in disease development and the elucidation of cellular processes have been, and continue to be, integral in the development of novel therapies and therapeutic approaches. Significant progress has been made in highlighting key biological pathways, some of which are shared with other autoimmune diseases. Evidence suggests a relationship between psoriasis and specific comorbidities, some with a confirmed biological basis and others, such as obesity, with intriguing but complex links to psoriasis. A new class of biologic agents selectively targeting interleukin (IL)-23p19 have shown additional efficacy in improving disease severity, even compared with those targeting both IL-12 and IL-23, highlighting the significance of IL-23 in psoriasis.1 The true relationships between comorbid conditions in patients with psoriasis and determination of the mechanisms involved will be important in the long-term management of the condition.
The Epidemiology and Inter-relation of Psoriasis and Other Interleukin-23- Related Diseases
Professor Alexa Kimball
Treatment Landscape of Patients with Psoriasis
Recognition of psoriasis as an immune-mediated disease has shaped the treatment landscape over the last several years, enabling targeted and effective therapies to be developed. Most recently, the elucidation of the role of IL-23 in psoriasis and other diseases has been a fascinating story of concurrent and synergistic discovery driven by laboratory findings, translational investigation, clinical observation and epidemiology. As has often been the case in dermatology, clinical observation has driven some of the early hypotheses. These key questions have resulted in clinical and laboratory observations revealing the effect of p40 inhibitors on both IL-12 and a relatively newly recognised cytokine, IL-23. This in turn ultimately revealed the novel T helper (Th)17 cell pathway and the pivotal role of IL-23 in the pathogenesis and treatment of psoriasis.
In 1986, Mosmann et al.2 observed that Th cells, differentiated from naïve CD4 T cells, could be separated into two classes, Type 1 (Th1) and Type 2 (Th2), depending on which specific cytokines were secreted in response to antigenic stimulation. Besides the classical Th1 and Th2, other subsets have also been identified, each with a specific cytokine profile, including Th3, regulatory T (Treg) cells, Th9 cells, and follicular Th cells.3,4 Human IL-17 and IL-23 were cloned in 19955 and 2000,6 respectively, with IL-17-producing CD4+ T cells characterised as a distinct set of cells termed Th17 or ThIL-17 cells in 2005.7,8 During this time, evidence accumulated to support the role of IL-23 in autoimmunity, the relationship between IL-23 and IL-17, and the importance of IL-23 in activating Th17 cells.9 In 2006, the importance of IL-23 in driving innate and T cell-mediated intestinal inflammation was identified, and the link between IL-23 mutations and Crohn’s disease considered.10,11 At this time, it was known that Th17 cells are primarily found in the gut and skin, and are involved in the gut barrier. Ustekinumab, developed as an IL-12p40-specific inhibitor, was in Phase II clinical trials before it was later discovered that the p40 subunit was shared by IL-12 and IL-23, enabling targeting of both cytokines with the same drug. Today, ustekinumab is approved for the treatment of plaque psoriasis, psoriatic arthritis (PsA), and Crohn’s disease.12,13
Several other antibodies targeting the Th17 pathway are available to treat psoriasis. These include the anti-IL-17 inhibitors secukinumab, ixekizumab, and brodalumab,14 and the anti-IL-23p19-specific inhibitor guselkumab.15 Other IL-23p19-specific inhibitors are also in development, such as tildrakizumab and mirikizumab.1,16,17 As a result, it is now possible to examine differences between the IL-17 and IL-23 pathways, and assess any beneficial effects of selective blockade throughout psoriasis and other rheumatic diseases. In addition, multiple single nucleotide polymorphisms associated with inflammatory bowel disease (IBD) have been identified in genes involved in the IL-23/IL-17 pathway.18 As such, multiple pathways with many nuances are involved in these autoimmune diseases. A better understanding of the role of the IL-17/IL-23 pathway in the pathogenesis of psoriasis and its comorbidities will form the basis of managing these conditions.
Psoriasis: Demographics and Comorbidities
Psoriasis is a relatively common chronic inflammatory skin disorder that affects at least 100 million people globally, with a reported prevalence in adults that ranges from 0.5% in the USA to 11.4% in Norway.19–21 Psoriasis is a significant public health challenge, affecting over 10-fold more people than IBD/Crohn’s disease, rheumatoid arthritis (RA), PsA, or multiple sclerosis.22–26 Using registry data, the differences in baseline characteristics between these diseases have been compared. The mean age of patients with psoriasis was around 50 years and just under 50% of patients were female.12,27 Notably, patients with psoriasis had a relatively high BMI, with a mean of approximately 30; 45–48% of patients were obese and 80% were overweight.12,27 In comparison, patients with RA were slightly older (mean age: 57.6 years) and more patients were female (76.7%).28 Patients with Crohn’s disease were younger than those with psoriasis, with a mean age of 40.3 years, and had a lower average BMI of 24.7.29
While the exact cause of psoriasis is unknown, triggers (e.g., injury to the skin, environmental and patient factors) can cause the release of proinflammatory cytokines (such as tumour necrosis factor [TNF]-α, IL-12, IL-17, and IL-23), resulting in recruitment of immune cells, decreased differentiation and increased proliferation of keratinocytes, and a sustained local inflammation at skin lesions.30 It has been proposed that when psoriasis is not controlled, proinflammatory cytokines are released into the circulation and precipitate systemic inflammation, which can induce insulin resistance, endothelial dysfunction, and cardiovascular diseases.30 As such, systemic inflammation may be the biological basis of a link between the pathophysiology of psoriasis and comorbid conditions such as obesity, hypertension, dyslipidaemia, and Type 2 diabetes mellitus.30
Other comorbid conditions associated with psoriasis include arthritis, cancer, IBD, and infections. PsA is a very common comorbidity that occurs in up to 30% of patients with psoriasis;31 however, the biologic relationship between the two is still being debated. The overlapping biology between psoriasis and IBD is, in some ways, clearer and, as can be seen in Table 1, patients with psoriasis are more likely to have Crohn’s disease (odds ratio [OR]: 2.49) than ulcerative colitis (OR: 1.64).32 The links between psoriasis and obesity or metabolic syndrome (as well as cardiovascular disease, cancer, and infection) are of great interest, and patients with psoriasis are more likely to have metabolic syndrome than people without psoriasis (OR: 2.26). The hazard ratio (HR) for end-stage renal disease is high (HR: 4.15),32 but the overall prevalence is relatively low (0.1% globally);33 therefore, the increased risk is comparatively low versus diseases such as hypertension (HR: 1.09),32 which affects 1 billion people worldwide.34 In addition to social stigma, distress, and discomfort, patients with psoriasis experience increased rates of depression (HR: 1.39), suicidal thoughts (HR: 1.44), and anxiety (HR: 1.31) compared with the general population.32 Even though a causal biological role in the aetiology and pathophysiology of major depression in patients with psoriasis remains to be identified, possible links have been made between increased levels of proinflammatory cytokines and depression.35 Future research will aim to further characterise relationships between psoriasis and important comorbidities such as those mentioned above.
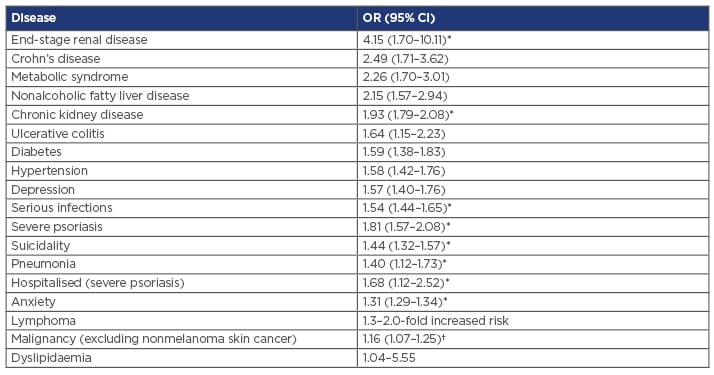
Table 1: Risk of comorbidities in patients with psoriasis compared with the non-psoriatic population.
*Hazard ratio. †Standardised incidence ratio.
CI: confidence interval; OR: odds ratio.
Adapted from Takeshita et al.32
It therefore appears that chronic inflammatory systemic diseases (e.g., IBD, PsA) may be linked to psoriasis through common pathogenic mechanisms, such as the proinflammatory cytokines TNF-α, IL-17, and IL-23.36-38 An understanding of the fundamental biology of psoriasis has been gained by observing in clinical practice the effects of new agents that target the IL23p19 moiety of IL-23.
Inflammatory Bowel Disease
The main forms of IBD are Crohn’s disease and ulcerative colitis. Classically, Crohn’s disease was thought to be a Th1-driven disease, and ulcerative colitis a Th2-driven condition. Even though clinical research focussed initially on IL-12, IL-23 was quickly identified as an important driver of IBD, based on the presence of both Th17 and Treg cells in conjunction with substantial inflammation, and the role of IL-23 in promoting Th17 and Treg survival (Figure 1).39 In addition, clinical trials of the IL-17 inhibitors brodalumab40 and secukinumab41 in Crohn’s disease patients were terminated due to a lack of efficacy and/or disease worsening. Post-hoc analyses in the secukinumab trial found that disease exacerbation was associated with an increase in inflammatory markers, including C-reactive protein and faecal calprotectin, which are targets of IL-22 and IL-23.41,42 It has been suggested that basal levels of IL-17 may confer a protective effect on the gut based on the finding that inhibition of IL-17 led to upregulation of IL-23 and retinoic acid receptor-related orphan receptor gamma t (RORγt)-dependent inflammatory cytokines, and corresponding mucosal tissue damage.42
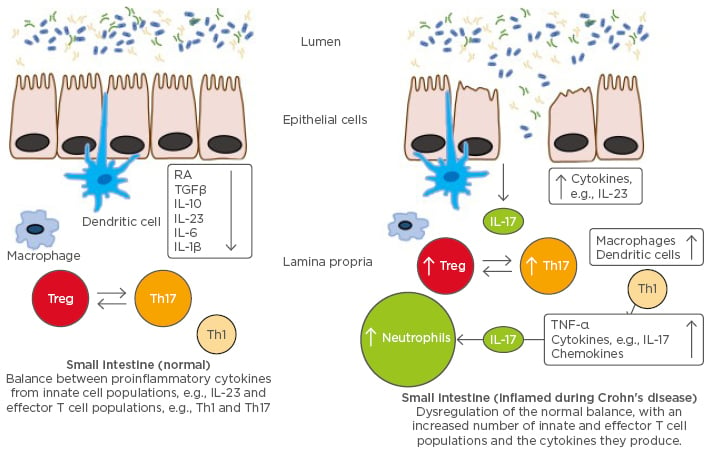
Figure 1: Cytokines in normal small intestine versus inflamed mucosa of patients with inflammatory bowel disease.
IFN: interferon; IL: interleukin; RA: retinoic acid; TGFβ: transforming growth factor beta; Th: T helper; TNF-α: tumour necrosis factor alpha; Treg: regulatory T cells.
Adapted from Abraham and Cho.39
Psoriatic Arthritis
Multiple studies have suggested opposing roles for IL-12 and IL-23 in the pathophysiology of PsA. One preclinical study in particular has shown that IL-23 deficient mice are generally protected against joint disease, whereas IL-12-deficient mice develop more severe joint inflammation.43 This finding suggested a potential role for IL-23 in the development of joint disease, while IL-12 may have more of a protective effect. For example, ustekinumab, an inhibitor of IL-12p40 and IL-23p40, was one of the first biological treatments developed for PsA as an alternative to anti-TNF therapy, but may not be as effective as TNF-α inhibition.44,45 Murine data suggest that IL-23p19 inhibition may have a theoretical advantage over ustekinumab in PsA.45 As our understanding of the pathophysiology of PsA develops, our therapeutic strategies will also improve.
Obesity and Metabolic Syndrome
While the biological relationship between psoriasis and obesity is unknown, clinical observations have suggested that obesity often precedes and increases the risk of onset of psoriasis.46 In addition, the IL-17/IL-23 axis may be involved in the pathogenesis of both obesity and diabetes.47,48 In a study of obese women, levels of IL-17 and IL-23 were elevated but did not correlate with the production of proinflammatory cytokines, macrophage migration inhibitory factor, or adipokine leptin.48 No link was identified between IL-17 and IL-23 levels and BMI or waist circumference. The authors concluded that increases in IL-17 and IL-23 are independent of increases in abdominal fat, insulin resistance, and leptin and macrophage migration inhibitory factor levels.48 However, the source of these cytokines was not identified, and it could be speculated that this phenomenon is part of the inflammatory cascade that is detrimental to patients with psoriasis. Interestingly, a further preliminary study in patients with psoriasis and metabolic syndrome found a positive correlation between IL-23 and fasting glucose (correlation coefficient [r]: 0.432; p<0.05), and, unexpectedly, a negative correlation between IL-23, IL-22, and IL-12 and waist circumference (r: -0.504, r: -0.556, and r: -0.511, respectively; p<0.05).49 Metabolism and the immune system appear to be intrinsically linked in complicated and sometimes paradoxical ways, and are important targets for future research.
Neoplasms and Anticipated Infection Risk
Experimental models have shown that, following inflammation, IL-12 inhibition promotes tumour initiation, growth, and metastasis via compromise to the Th1 antitumour response. Conversely, IL-23 inhibition does not appear to promote tumour development,50 and elevated levels of IL-23 have been shown in both lung cancer51 and colon cancer.52 Given that co-depletion of IL-12 and IL-23 can result either in tumour formation or resolution of inflammation, further investigation into a potential synergistic effect is warranted. Treatment with ustekinumab was not associated with an increased malignancy risk among 12,090 patients in the Psoriasis Longitudinal Assessment and Registry (PSOLAR).53 In contrast, longer term (≥12 months) treatment with a TNF-α inhibitor was associated with an increased risk of malignancy (OR: 1.54; 95% confidence interval [CI]: 1.10-2.15; p=0.01).53 To date, large Phase III studies with p19-specific inhibitors, guselkumab and tildrakizumab, have had similar results in terms of reported malignancies, with no new safety signals identified.54,55
In terms of predicted infection risk, candidiasis is expected and evident with IL-17 inhibition, whereas no candidiasis or Staphylococcus aureus infection risk has been documented in relation to IL-23p19 inhibition.56 No other unusual safety signals have been observed in these early studies.56
Summary
IL-23 appears to be a common component for the pathogenesis of psoriasis and the chronic inflammatory systemic diseases described above. Better understanding the role of IL-23 in the pathogenesis of psoriasis and its associated comorbidities will be critical to the long-term management of the disease.
Future Thoughts
There are many unanswered and intriguing questions in relation to comorbidities associated with psoriasis, such as depression, anxiety, inflammation, and multiple sclerosis. While we are improving the symptoms of psoriasis, we are not eradicating these completely, so there is still a substantial emotional burden for patients. Multiple sclerosis also remains a very interesting area, with further developments expected. In this rapidly evolving therapeutic field we are progressing in leaps and bounds towards truly understanding the biology of the disease and improving patient quality of life.







