Presenters: Pedro A. Laires,¹ Jonathan A. Bernstein,² Ana Giménez-Arnau,³ Marcus Maurer⁴
1. Novartis Pharma AG, Basel, Switzerland
2. University of Cincinnati College of Medicine and Bernstein Clinical Research Center, Cincinnati, Ohio, USA
3. Dermatology Department, Hospital del Mar, IMIM, Universitat Autònoma, Barcelona, Spain
4. Dermatogical Allergology, Allergie-Centrum-Charité, Department of Dermatology and Allergy, Charité – Universitätsmedizin, Berlin, Germany
Disclosure: Laires is an employee of Novartis Pharma AG, Basel, Switzerland. Bernstein reports grants and personal fees from Novartis, Astra Zeneca, Allakos, Genentech, Sanofi Regeneron, Celldex, and Amgen outside the submitted work. Giménez-Arnau reports roles as a medical advisor for Uriach Pharma, Sanofi and Genentech, Novartis, FAES, GSK, AMGEN, and Thermo Fisher; has research grants supported by Uriach Pharma, Novartis, and Instituto Carlos III- FEDER; and also participates in educational activities for Uriach Pharma, Novartis, Genentech, Menarini, LEO- PHARMA, GSK, MSD, Almirall, AVENE, and Sanofi. Maurer is or recently was a speaker and/or advisor for and/or has received research funding from Amgen, Allakos, Aralez, AstraZeneca, Celldex, FAES, Genentech, GIInnovation, Kyowa Kirin, Leo Pharma, Menarini, Novartis, Moxie, MSD, Roche, Sanofi, Third Harmonic, UCB, and Uriach.
Acknowledgements: Medical writing assistance was provided by Jennifer Taylor, London, UK.
Support: The publication of this article was funded by Novartis. The views and opinions expressed are those of the authors and not necessarily those of Novartis.
Citation: EMJ Dermatol. 2021;9[1]:35-41.
Meeting Summary
The latest international guidelines on urticaria were published in September as a joint initiative from the European Academy of Allergology and Clinical Immunology (EAACI), the Global Allergy and Asthma European Network (GA²LEN), the European Dermatology Forum (EDF; EuroGuiDerm), and the Asia Pacific Association of Allergy, Asthma and Clinical Immunology (APAAACI).1 The guidelines state that the goal of treatment is to treat the disease until it is gone and as efficiently and safely as possible aiming for a continuous weekly Urticaria Activity Score (UAS7) of 0, complete control, and a normalisation of quality of life. It is recommended that in addition to healing the disease and reducing disease activity, the therapeutic approach to chronic urticaria (CU) should involve using patient-reported outcome measures (PROMs), such as the Urticaria Activity Score (UAS), UAS7, the angioedema activity score (AAS), the chronic spontaneous urticaria quality of life questionnaire (CU-Q2oL), the angioedema quality of life questionnaire (AE-QoL), the urticaria control test (UCT), and the angioedema control test (AECT). Complete urticaria control is required for the least impact on quality of life, sleep, activity, and work. This was demonstrated in three posters presented at the European Academy of Dermatology and Venereology (EADV) 2021 Congress in Vienna, Austria. An analysis of real-world data from the “A World-wide Antihistamine-Refractory chronic urticaria patient Evaluation” (AWARE) study demonstrated that patients with chronic spontaneous urticaria (CSU) achieving UAS7=0 had significantly lower mean Dermatology Life Quality Index (DLQI) and CU-Q2oL scores compared with patients in other disease activity bands, including the well-controlled state (UAS7=1–6). A further analysis of the AWARE study data showed that patients achieving UAS7=0 had significantly lower mean CU-Q2oL sleep domain scores compared with patients in other UAS7 disease activity bands. Patients achieving complete control (UAS7=0) even had significantly lower CU-Q2oL sleep domain scores compared with well-controlled (UAS7=1–6) patients. Real life data from the AWARE study have shown that a large proportion of patients do not reach complete control and new treatment options are needed. An analysis of pooled data from a Phase IIb ligelizumab study found that complete control was also associated with less interference with activity and work.2 Comparing patients with UAS7=0 (urticaria free) to those with UAS7=1-6 (well-controlled), there was a 178-times higher likelihood of having a Weekly Activity Interference Score (AIS7)=0 and a three-times higher likelihood of having an Overall Work Impairment Score=0, confirming the findings from AWARE Further analysis of the AWARE study suggested the advantages of early and sustained high-efficacy treatment on the quality of life of patients with CSU. Mean DLQI scores at baseline and Year 1 in the early omalizumab treatment group were 12.3 and 2.9, respectively, compared with 11.6 and 4.1 in the late group, and 8.4 and 4.5 in the no omalizumab group. Furthermore, early and sustained use of omalizumab was significantly associated with achieving a DLQI score of 0–1 at 1 year of follow-up in the AWARE study, after adjustment for other relevant factors. To assess the holistic effect of treatment in patients with CSU, composite scores were created combining symptoms with quality of life. A composite score of Weekly Hives Severity Score (HSS7), Weekly Itch Severity Score (ISS7), and Weekly Angioedema Activity Score (AAS7) was used to examine complete control in the Phase IIb ligelizumab study. When compared to omalizumab, patients in the ligelizumab arms had a 15.9–19.4% higher chance of achieving CSU completely controlled status. When compared to placebo, patients on ligelizumab had a 36.2–39.7% higher chance of achieving CSU completely controlled status. CSU disease activity can fluctuate and because UAS7 is a weekly score it may not adequately record information on flare-ups. A novel rolling UAS7 (rUAS7) was used to define flare-ups in an exploratory analysis of the Phase IIb ligelizumab study. During the treatment period, the proportion of patients who did not experience flare-ups was 50.0%, 58.8%, 47.0%, and 27.9% for ligelizumab 72 and 240 mg, omalizumab 300 mg, and placebo, respectively.Introduction
Disease control or activity should be assessed in trials and in clinical practice. The guidelines state that “treatment should follow the basic principles of treating as much as needed and as little as possible taking into consideration that the activity of the disease may vary. This implies stepping up or stepping down in the treatment algorithm according to the course of disease following the principle assess, adjust, act, and reassess.” In this context, UCT <12 defines uncontrolled disease and requires a step-up in treatment. Patients with UCT=12–15 have well-controlled disease and the action is to continue therapy and try to optimise. Those with UCT=16 have completely controlled disease and a step-down (i.e., reducing dose or extending intervals) is indicated based on individual factors.
Three posters presented at EADV 2021 showed that in patients with CSU, complete control was correlated with better health-related quality of life (HRQoL),3 better sleep quality,4 and less interference with activity and work.5 The advantages of early and high-efficacy treatment on quality of life were also outlined.6 A more holistic assessment of treatment effect using a composite score combining symptoms and quality of life showed that more patients achieve complete control with ligelizumab 240 or 72 mg compared to omalizumab 300 mg.7 Finally, a novel exploratory analysis examined the stability of complete response to anti-IgE treatments, once achieved.8
Benefits of Complete Control on Quality of Life, Sleep, Activity, and Work3,4,5
Pedro A. Laires, Jonathan A. Bernstein
Three posters presented at EADV 2021 suggested that in patients with CSU, complete control was correlated with better HRQoL,3 better sleep quality,4 and less interference with activity and work.5 All three studies showed that compared with complete control, those with even one disease activity band higher (i.e., well-controlled) experienced a significantly greater burden of their condition on PROMs.
An analysis of real-world data from the AWARE study demonstrated that patients achieving UAS7=0 had significantly (p<0.0001) lower mean DLQI and CU-Q2oL scores compared with patients in other disease activity bands, indicating better HRQoL in those with complete symptom control. Figure 1 shows the CU-Q2oL scores by UAS7 categories. The Pearson correlation coefficient between the DLQI and UAS7 score was 0.63 (p<0.0001) and between the CU-Q2oL and UAS7 score was 0.57 (p<0.0001), indicating a significant positive correlation between symptom control and HRQoL.
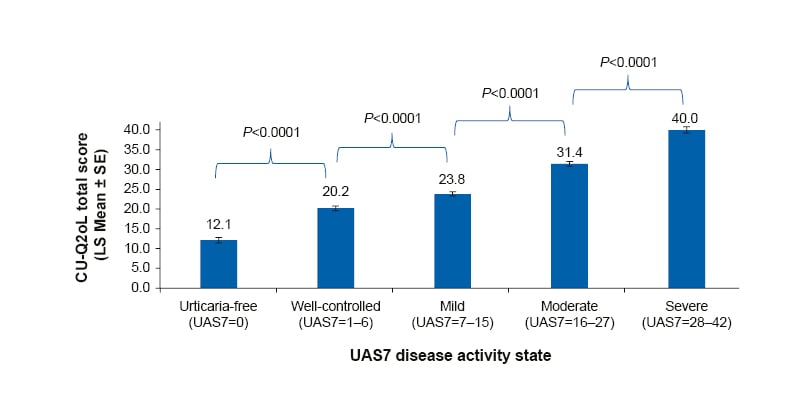
Figure 1: CU-Q2oL total score by UAS7 disease states.
For this analysis, data from patients across all timepoints up to Year 1 were considered.
CU-Q2oL: Chronic Urticaria Quality of Life questionnaire; LS: least squares; SE: standard error; UAS7: weekly Urticaria Activity Score.
Figure 2 shows the correlation between the CU-Q2oL sleep domain and UAS7 bands. Patients achieving UAS7=0 had significantly (p<0.0001) lower mean CU-Q2oL sleep domain scores compared with patients in other UAS7 disease activity bands, indicating improved quality of sleep in patients with complete symptom control. The Pearson correlation coefficient between the CU-Q2oL sleep domain and UAS7 score was 0.45 (p<0.0001), indicating a significant positive correlation between symptoms and sleep. The analysis also demonstrated that the CU-Q2oL sleep domain score correlated with DLQI status. Patients achieving DLQI=0–1 had significantly (p<0.0001) lower mean CU-Q2oL sleep domain scores compared with patients with higher DLQI scores.
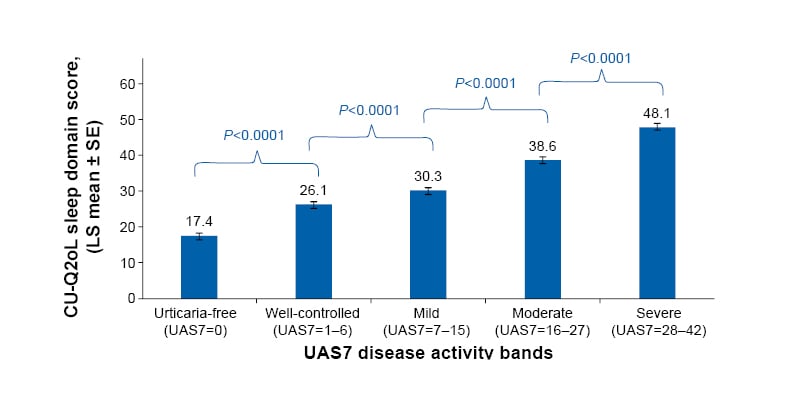
Figure 2: CU-Q2oL sleep domain scores by UAS7 bands from baseline up to Year 1.
For this analysis, data from patients across all timepoints up to Year 1 were considered.
CU-Q2oL: Chronic Urticaria Quality of Life questionnaire; LS: least squares; SE: standard error; UAS7: weekly Urticaria Activity Score.
An analysis of pooled data from the Phase IIb ligelizumab study9 found that in patients with UAS7=0, 91.1% reached DLQI=0–1 simultaneously. When patients with UAS7=0 were compared to those with UAS7=1–6, there was a five-times higher (odds ratio [OR]: 5.37; 95% confidence interval [CI]: 3.2–9.1; p<0.0001) likelihood of having DLQI=0–1. For SIS7, in patients with UAS7=0, 99.7% reached SIS7=0 simultaneously. Comparing patients with UAS7=0 to those with UAS7=1–6, there was a 137-times higher (OR: 136.77; 95% CI: 37.2–502.8; p<0.0001) likelihood of having SIS7=0. Regarding AIS7, in patients with UAS7=0, 99.7% simultaneously reached AIS7=0. Comparing patients with UAS7=0 to those with UAS7=1–6, there was a 178-times higher (OR: 178.11; 95% CI: 29.2–1,085.8; p<0.0001) likelihood of having AIS7=0. Finally, 85.3% of patients with UAS7=0 reached an Overall Work Impairment Score=0. Comparing patients with UAS7=0 to those with UAS7=1–6, there was a three-times (OR: 3.04; 95% CI: 1.8–5.1; p<0.0001) higher likelihood of having Overall Work Impairment Score=0.
Benefits of Early Treatment on Quality of Life6
Pedro A. Laires
Previous studies have demonstrated the effectiveness and safety of omalizumab in CSU,10-12 but real-world evidence describing the role of early treatment is limited. This analysis of the real-world AWARE study investigated HRQoL in CSU patients undergoing early and sustained omalizumab treatment.
Early and late treatment was defined as switch to omalizumab at 3 and 6–9 months from baseline, respectively, and continued treatment up to 1-year follow-up. Mean DLQI scores at baseline and Year 1 were assessed in patients with early, late, and no omalizumab treatment (no switch to omalizumab during the study).
Early and sustained use of omalizumab was significantly associated with achieving DLQI=0–1, with an OR of 2.4 (95% CI: 1.5–3.8; p<0.001). Despite a positive trend, no significant association was seen with late treatment (OR: 1.5; 95% CI: 0.8–2.6; p=0.174) or overall omalizumab treatment for achieving DLQI=0–1 (OR: 1.6; 95% CI: 0.8–3.2; p=0.160).
In summary, the results indicate that early and sustained treatment with omalizumab in a real-world setting is associated with improved quality of life in patients with CSU. The authors said the findings highlight the importance of early access and use of high-efficacy treatments in the management of CSU, whenever clinically required, to attain the best possible outcomes for patients.
A Composite Score Combining Symptoms with Quality of Life to Evaluate Complete Control of Urticaria with Ligelizumab7
Ana Giménez-Arnau
Assessing the holistic effect of treatment in patients with CSU requires evaluating different patient-reported outcomes (PROs) such as the HSS7, ISS7, AAS7, and DLQI. While these measures correlate, patients may not always exhibit the same magnitude of response for each PRO and there may be lags between responses. In addition, not all patients experience angioedema, which can severely impact quality of life as well. This study therefore examined complete urticaria control using composite scores of different PROs. Patients were considered to have CSU completely controlled if they recorded concurrent HSS7=0, ISS7=0, and AAS7=0, and to be CSU-free if they also recorded DLQI=0–1.
Table 1 shows the proportion of patients in each of the four treatment arms who achieved complete responses in the PROs and who were completely controlled or CSU-free according to the composite scores. The authors stated that the composite outcome evaluation provided a more holistic approach to the treatment response and clearly differentiated outcomes across treatment arms.
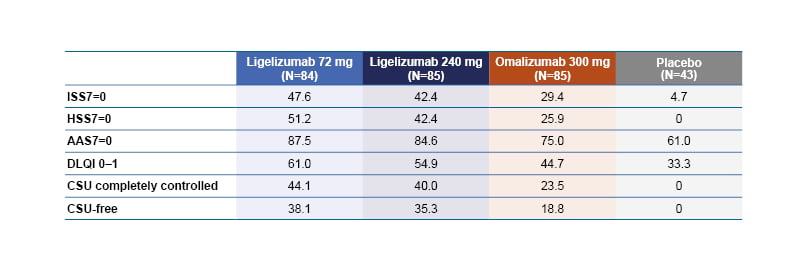
Table 1: The percentage of patients who achieved complete response in the HSS7, ISS7, AAS7, and DLQI 0–1 at Week 12.
Data were analysed using non-responder imputation. CSU completely controlled: free from signs and symptoms of urticaria with concurrent HSS7=0, ISS70, and AAS7=0; CSU-free: CSU completely controlled with concurrent DLQI=0–1.
AAS7: Weekly Angioedema Activity Score; CSU: Chronic Spontaneous Urticaria; DLQI: Dermatology Life Quality Index; HSS7: Weekly Hives Severity Score; ISS7: Weekly Itch Severity Score.
The investigators estimated the probability of achieving CSU completely controlled and CSU-free status by treatment at Week 12. When compared to omalizumab, patients in the ligelizumab arms had a 15.9–19.4% higher chance of achieving CSU completely controlled status, and 15.7–18.2% higher chance of achieving CSU-free status according to the composite scores. When compared to placebo, patients on ligelizumab had a 36.2–39.7% higher chance of achieving CSU completely controlled status, and 32.0–34.5% higher chance of achieving a CSU-free status.
To summarise, in the core Phase IIb and extension studies, ligelizumab was more likely to achieve and sustain complete control concurrently on all PROs compared with omalizumab or placebo in patients with CSU. The authors concluded that using a composite score of validated PROs for CSU could be useful in clinical studies for differentiating response to treatments.
[/hl]Ligelizumab Shows Good Stability of Response in Patients with Chronic Spontaneous Urticaria: A Novel Exploratory Analysis of Urticaria Flare-ups8 [/hl]
Marcus Maurer
CSU can present with intermittent exacerbations of disease activity (flare-ups) even in patients undergoing treatment. The UAS7 is used to assess the effect of treatment but lacks the granularity of daily assessment of urticaria activity, while daily UAS does not provide a stable overview of the trends in disease activity.
The current exploratory analysis used a novel rUAS7 to provide a stable assessment of disease activity while maintaining granularity. The study defined and measured flare-ups using a trend of continuous increase in rUAS7≥10 and explored the effect of ligelizumab treatment on flare-ups in patients with CSU.
The study included patients in the ligelizumab 72 mg and 240 mg, omalizumab 300 mg, and placebo treatment arms of the Phase IIb ligelizumab study. The rUAS7 was calculated as the sum of UAS for a set of 7 consecutive days starting on a given day. It was calculated for every possible set of 7 consecutive days during the study. A flare-up was defined as a temporary continuous increase of rUAS7≥10 (based on minimum important difference for UAS713,14) from the lowest rUAS7 achieved before the flare. The day when such a difference was <10 points from the previous minimum was considered to be the end of the flare-up. The number of days, including the first day, spent with a flare-up was calculated as the duration of the flare-up.
The number of flare-ups and time to first flare-up, as well as the proportion of flare-up free days, were evaluated for patients in each treatment arm during the 20-week treatment period and the first 12 weeks of the treatment-free follow-up period.
Regarding the number of flare-ups during the treatment period, the proportion of patients who did not experience flare-ups was 50.0%, 58.8%, 47.0%, and 27.9% for ligelizumab 72 and 240 mg, omalizumab, and placebo, respectively. The time to achieve a median rUAS7=0 (urticaria free) with ligelizumab 72 mg, 240 mg, and omalizumab was 48, 71, and 101 days (the placebo arm did not reach the median value for rUAS7=0). Regarding the estimated time to first flare-up in 25% of patients, this was 36 and 47 days for ligelizumab 72 and 240 mg, respectively, 37 days for omalizumab, and 31 days for placebo.
In summary, treatment with ligelizumab showed good control of multiple flare-ups and disease stability. The authors concluded that assessment of flare-ups could improve CSU management in clinical practice.
Conclusion
Clinical trials and real-world data demonstrate the benefits and importance of achieving complete control in patients with CSU on quality of life, sleep quality, and ability to work, and illustrate the advantages of early treatment. Novel assessments may be used in future to better reflect the burden of disease, and new treatment options promise to improve patient wellbeing.







